Unlocking the Hidden Potential of Mesenchymal Stem Cells: Decoding the Trilineage Differentiation and Key Applications
Nov 04,2025
Mesenchymal stem cells (MSCs) are adult stem cells. With their unique biological characteristics and remarkable therapeutic potential, MSCs have become a rising star in the field of disease research. As of May 2024, there were more than 1,200 ongoing clinical trials involving MSCs worldwide, and over 27 MSC-related products had been approved, providing preliminary evidence of their clinical value.
To help readers quickly and systematically grasp the essential knowledge of MSCs, we have planned a series of posts covering the following topics in sequence: an overview of MSCs, in vitro culture and expansion, trilineage differentiation, and applications in cell therapy.
In this issue of Cell Culture Academy, we examine MSC trilineage differentiation to elucidate its underlying mechanisms and potential applications.
I. What Is Trilineage Differentiation?
MSCs are multipotent, with the most representative differentiation pathways being adipogenic, osteogenic, and chondrogenic. These three lineages are referred to as trilineage differentiation.
Recent studies have explored the molecular basis of this process, focusing on transcriptional regulation, signaling pathways, and epigenetic mechanisms. However, the precise regulatory mechanisms remain unclear, and how the three lineages maintain their balance and influence one another remains a key topic of ongoing research.
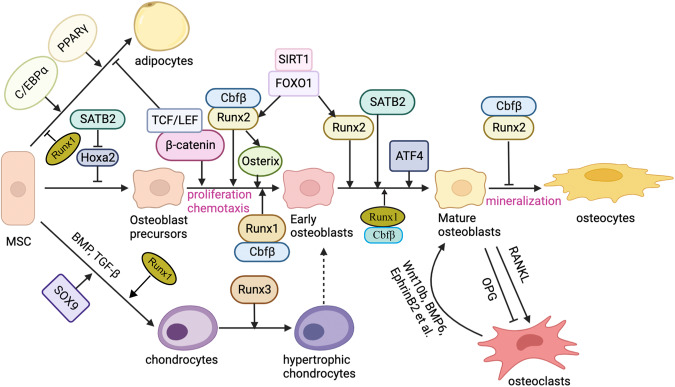
Figure 1. Roles of signaling pathways and key factors in MSC differentiation
Image reproduced from [1]
II. Analysis of Trilineage Differentiation
1. Osteogenic Differentiation
Osteogenic differentiation represents a critical process in bone formation. Under the coordinated influence of cytokines such as BMPs and TGF-β, as well as hormones and physicochemical factors (e.g., calcium ion concentration and mechanical stress), MSCs first differentiate into pre-osteoblasts and enter a phase of rapid proliferation. As proliferation gradually decreases, genes associated with extracellular matrix (ECM) maturation become increasingly activated. During this stage, osteoblasts synthesize and secrete large amounts of organic matrix, primarily composed of type I collagen, forming the osteoid. A hallmark of this phase is the elevated activity of alkaline phosphatase (ALP), an early biochemical indicator of osteogenic differentiation. As osteoblasts mature, they further express ECM calcification-associated proteins, including osteocalcin (OCN) and osteopontin (OPN), which result in significantly enhanced mineralization capacity.
2. Adipogenic Differentiation
Adipose tissue consists of white and brown adipose tissue, both of which originate from MSCs but differ in their regulatory mechanisms of differentiation. Adipogenic differentiation of MSCs generally proceeds through three sequential stages. First, MSCs commit to the preadipocyte lineage; second, preadipocytes undergo clonal expansion; and finally, the cells enter terminal differentiation, forming mature adipocytes capable of accumulating lipid droplets. This process is primarily regulated by the transcription factors PPARγ and C/EBPα, which play central roles in the transcriptional network governing adipogenesis.
3. Chondrogenic Differentiation
Chondrocytes are responsible for the formation and maintenance of cartilage tissue, and their microenvironment exerts a profound influence on their function and homeostasis. Under the regulation of signaling pathways such as TGF-β and BMP, MSCs first aggregate to form cartilage nodules and differentiate into prechondrocytes. These prechondrocytes secrete type II collagen and proteoglycans to establish the cartilage matrix, while continuing to deposit matrix components during subsequent differentiation stages. Ultimately, they mature into functional chondrocytes capable of maintaining cartilage integrity and biomechanical properties.
Bone homeostasis: Continuous renewal of bone tissue relies on a balance between osteoclast-mediated bone resorption and new bone formation by osteoblasts. Osteogenic differentiation of MSCs is a key determinant of bone mass maintenance and the preservation of bone microstructural integrity.
Articular cartilage repair and homeostasis: The chondrogenic differentiation potential of MSCs contributes to cartilage repair and supports the homeostasis of the joint microenvironment. In adult articular cartilage, daily homeostasis is primarily maintained by resident chondrocytes.
Local adipose tissue homeostasis: Adipogenic differentiation of MSCs plays a role in sustaining the homeostasis of local adipose tissue, preventing abnormal fat accumulation and associated metabolic disorders. The direct influence of MSCs on systemic fat metabolism, however, remains to be fully elucidated.
Regulation of the tissue microenvironment: MSCs secrete a range of cytokines through paracrine signaling, modulating immune responses and participating in the maintenance and repair of the local tissue microenvironment.
3. Pathological Correlation and Disease Mechanism Research
The imbalance of MSC differentiation is closely associated with various diseases.
Abnormal osteogenic/adipogenic balance: When the differentiation of bone marrow MSCs toward the adipose lineage increases or osteogenic differentiation is inhibited, bone formation decreases, which can easily lead to osteoporosis, abnormal osteogenesis, and age-related bone loss.
Impaired chondrogenic differentiation: Insufficient chondrogenic differentiation restricts the production of cartilage matrix, thereby promoting the development of articular cartilage degeneration and osteoarthritis.
Studying the differentiation of MSCs under disease conditions helps to elucidate the pathological mechanisms and provides a theoretical basis for the repair and regeneration of bone, cartilage, and adipose tissues.
4. Applications in Regenerative Medicine and Tissue Engineering
The trilineage differentiation potential of MSCs makes them ideal seed cells for tissue engineering and regenerative medicine.
Osteogenic lineage: MSCs are utilized for bone defect repair, the construction of artificial bone, and the development of bone regeneration scaffolds.
Chondrogenic lineage: MSCs are applied in cartilage injury repair and in cell-based therapies for degenerative joint diseases.
Adipogenic lineage: MSCs are employed for adipose tissue reconstruction and in aesthetic medicine.
Moreover, standardized in vitro trilineage differentiation systems can serve as disease models for drug screening and for validating intervention targets in osteoporosis, obesity, and cartilage degenerative disorders, thereby providing an experimental foundation for the development of novel therapeutic strategies.
5. Individualized and Precision Medicine
Through directed differentiation, MSCs can be induced to generate specific functional cell types (e.g., osteoblasts, chondrocytes, or adipocytes), which can then be transplanted into damaged tissues to achieve targeted repair and regeneration.
Notably, patient-derived MSCs can be used for autologous transplantation, reducing the risk of immune rejection and enhancing repair efficacy. This approach offers new avenues and practical solutions for individualized and precision medicine.
IV. In Vitro Induction Examples of Trilineage Differentiation
In vitro induction of MSC trilineage differentiation provides critical insights into their differentiation mechanisms and offers a reliable reference for both basic research and clinical applications.
Pricella® has long been dedicated to cellular research, establishing a robust technical platform and accumulating extensive expertise in differentiation protocols. Leveraging this experience, we offer comprehensive solutions for trilineage induction and differentiation of MSCs derived from various sources, thereby supporting advanced research and translational applications.
Figure 2. Identification of trilineage differentiation of MSCs from various sources
References
[1] Zhu S, Chen W, Masson A, et al. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discovery. 2024 Jul 2;10(1):71.
[2] Li, J., Wu, Z., Zhao, L. et al. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Research & Therapy. 2023, 14, 381.
[3] Han X, Liao R, Li X, et al. Mesenchymal stem cells in treating human diseases: molecular mechanisms and clinical studies. Signal Transduction and Targeted Therapy. 2025, 10, 262.
Prev: Accutase Cell Detachment Solution: Gentle on Cells, Powerful in Performance

.jpg)
.jpg)
.png)
.png)
.jpg)
.jpg)

