A No-mistake Guide to High-activity Rat Articular Chondrocyte Isolation
Dec 30,2025
Articular chondrocytes are the sole cellular component of articular cartilage. They regulate extracellular matrix metabolism, preserve cartilage architecture, and maintain the local microenvironment, acting as the key "guardians" of joint health. They also serve as a classic model for studying cartilage repair, osteoarthritis mechanisms, and drug screening.
However, the dense matrix, compact structure, and avascular nature of cartilage make cell isolation and in vitro culture technically challenging and highly demanding.
In this edition of Cell Culture Academy, we provide a systematic, step-by-step guide to the isolation, identification, and culture of rat articular chondrocytes, enabling researchers to efficiently obtain primary cells with high viability and stable phenotypes.
I. Basic Information
Cell Name: Rat articular chondrocytes
Tissue Source: Articular cartilage
Cell Introduction:
Articular cartilage is a form of hyaline cartilage characterized by a smooth, pale-blue, glossy surface. It is composed of specialized collagen fibers that provide elasticity and load-bearing capacity. Chondrocytes reside within this collagenous matrix. From the superficial to the deep zones, cell morphology transitions from flattened to elliptical or round. Chondrocytes are responsible for maintaining the metabolic homeostasis of articular cartilage.
Morphological Characteristics:
Immature articular chondrocytes are located in the superficial zone and are individually distributed, relatively small in size, and elliptical, with their long axis parallel to the cartilage surface. With increasing depth, cell size increases and becomes round. The nucleus is round to oval with light staining, and the cytoplasm is weakly basophilic, often containing varying numbers of lipid droplets.
Mature chondrocytes typically form clusters of 2-8 cells within the cartilage lacunae. Originating from the proliferation of a single progenitor cell, these clusters are referred to as isogenous groups.
Under electron microscopy, articular chondrocytes exhibit surface protrusions and folds. The cytoplasm contains abundant rough endoplasmic reticulum, a well-developed Golgi apparatus, and a small number of mitochondria. In histological sections, chondrocytes often appear retracted with irregular contours, leaving a conspicuous pericellular space between the cell and its surrounding capsule.
II. Operational Procedures
1.Dissection Procedures
Select 14-day-old rats of strains such as Wistar or SD. After euthanasia using an overdose of sodium pentobarbital or cervical dislocation, secure the rat on a clean bench.
Incise the skin along the medial thigh and peel it downward to approximately 2 cm below the knee to fully expose the joint.
Identify the white patella, make a circumferential incision around it, separate the connective tissue, open the joint cavity, and remove residual tissue (Figure 1).
Excise the white cartilage layer at the femoral-knee junction (Figure 2). After washing, collect the cartilage tissue (Figure 3).
Carefully remove any attached fat, synovium, fascia, or inadvertently cut bone fragments, preserving only the white, translucent cartilage. Cut each piece of articular cartilage into 4-6 evenly sized fragments.
2.Tissue Digestion
Add the tissue fragments to the specialized digestive solutionn and digest until they are fully dissociated.
Terminate the digestion by adding complete culture medium, then filter, wash, and resuspend the cells to obtain a single-cell suspension.
3.Cell Culture and Subculture
After resuspending the cells in complete medium for rat articular chondrocytes, seed them into a T25 culture flask and incubate at 37℃ with 5% CO2 for 3-5 days under static conditions.
When cells reach 80-90% confluence, rinse them and digest for 1-3 minutes. Once cells round up under the microscope, add 3-5 mL of complete medium to stop digestion and gently pipette to disperse the cells.
For detailed procedures, refer to the official Rat Articular Chondrocyte Isolation and Culture Kit instruction manual.
III. Cell Identification
Morphology: Cells adhere well and exhibit a spindle-shaped or polygonal morphology under an optical microscope.
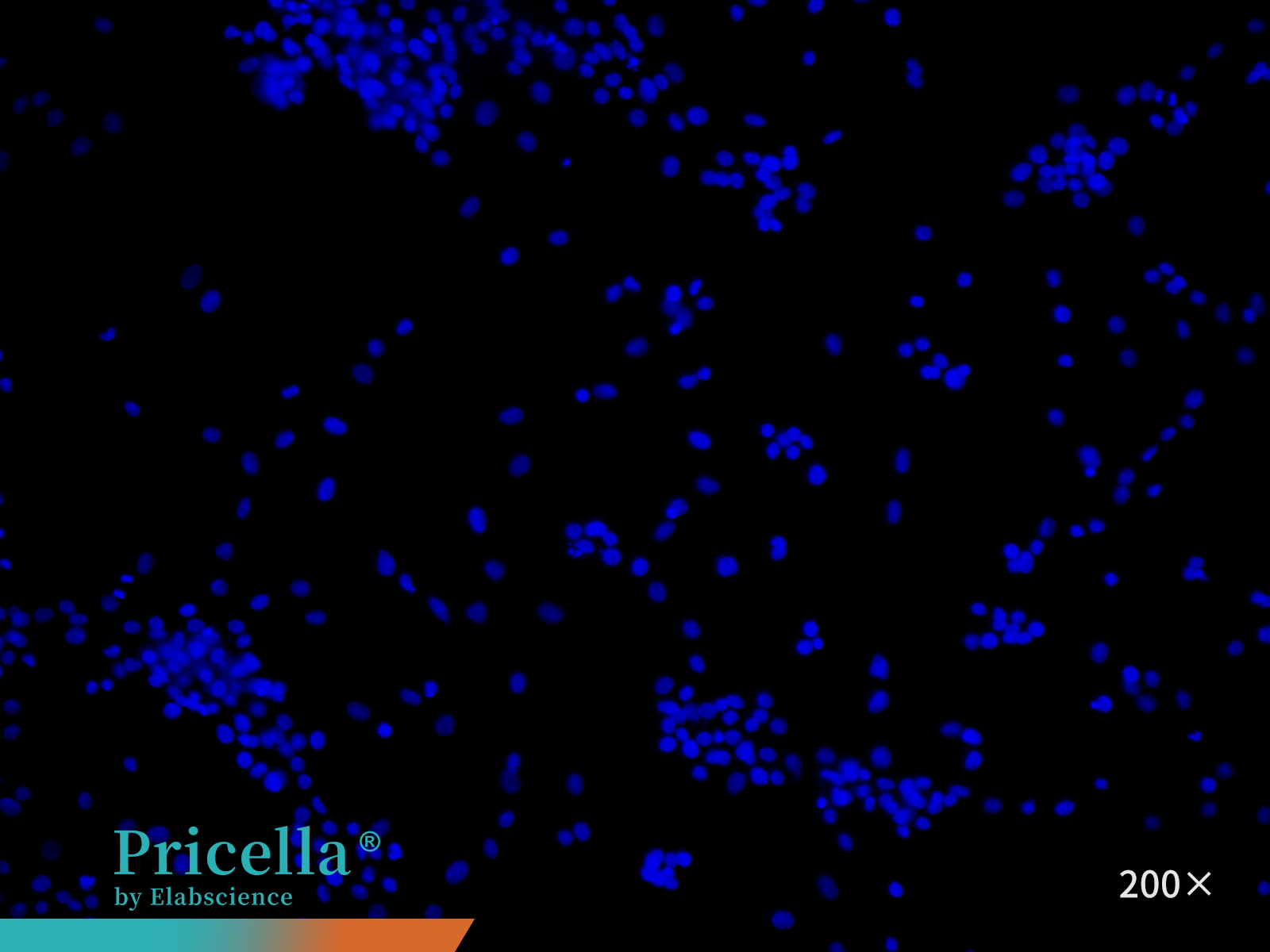
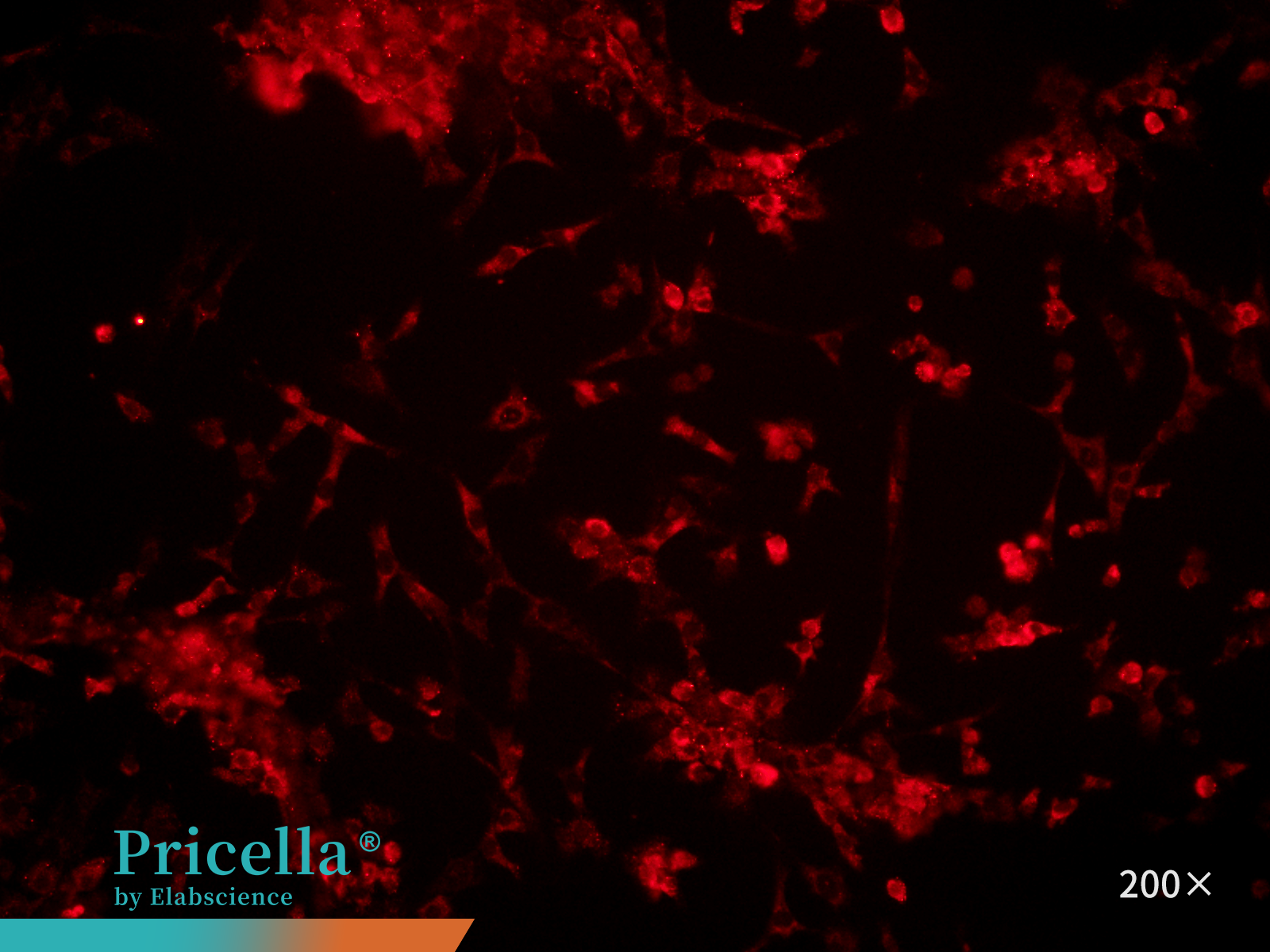
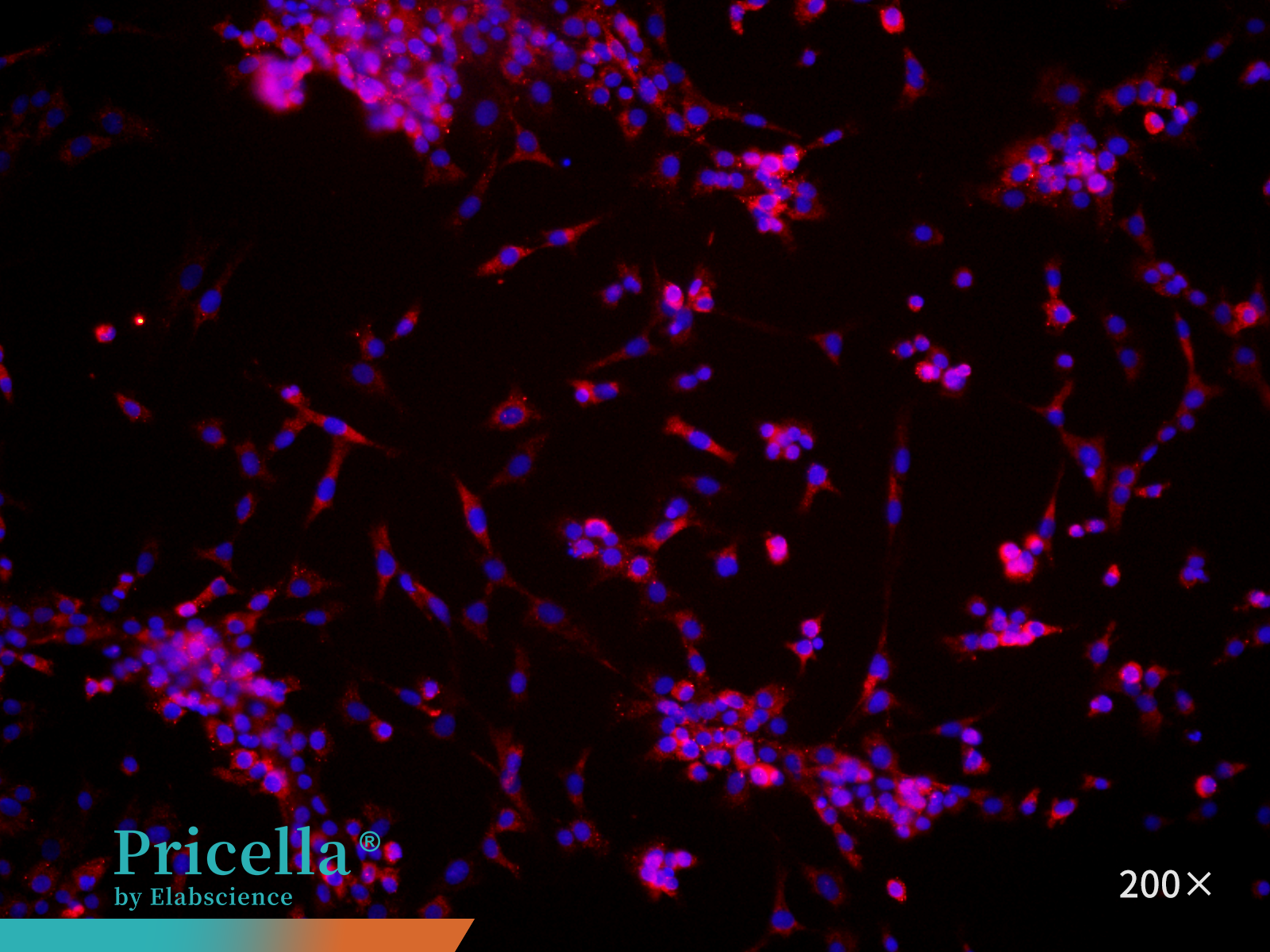
Marker Expression: Perform immunofluorescence staining using Collagen II as the primary antibody. Examine the cells under a fluorescence microscope (Figure 4). Purity typically exceeds 90%.
Figure 4. Immunofluorescence identification of rat articular chondrocytes
IV. Culture Information
Culture medium: Rat Articular Chondrocyte Complete Medium (including FBS, growth supplements, penicillin, streptomycin, etc.)
Medium change frequency: Every 3-4 days
Growth characteristics: Adherent growth
Passage ratio: 1:2
Passage characteristics: Can be passaged up to 5 times; optimal conditions are within passage 3
Digestive solution: 0.25% Trypsin Solution
V. Applications
Chondrocytes are crucial for in vitro studies of cartilage biology and have broad applications in joint disease research, drug screening, toxicology, tissue engineering, and inflammatory or immune regulation.
1.Disease mechanism studies: Model pathological conditions in vitro by exposing chondrocytes to inflammatory or mechanical stimuli. Monitor cellular and molecular responses to investigate the mechanisms underlying joint diseases.
2.Drug screening and toxicology: Use primary chondrocytes’ in vivo-like characteristics to establish cell-based screening systems. Evaluate drug effects on cartilage protection or repair, as well as potential chemical toxicity, providing reliable preclinical data.
3.Cartilage metabolism and tissue engineering: Exploit chondrocytes’ matrix-synthesis capacity with biomaterials to construct in vitro cartilage models, supporting studies on cartilage repair.
4.Inflammation and immune research: Establish co-culture systems of chondrocytes with synovial fibroblasts or macrophages to study intercellular interactions and inflammatory regulation in arthritis models.
Prev: Your Complete Guide to Isolating Bone Marrow Monocytes
Next: Key Tips for Isolating Rat Pulmonary Great Artery Endothelial Cells

.png)
.png)
.png)
.png)