Key Tips for Isolating Rat Pulmonary Great Artery Endothelial Cells
Jan 05,2026
The pulmonary great artery is the principal vessel linking the right ventricle to the pulmonary circulation. endothelial cells in this vessel are directly exposed to high-pressure blood flow and elevated shear stress, making them a critical in vitro model for studying cardiopulmonary vascular diseases. However, their limited availability and close association with smooth muscle cells increase the risk of contamination during isolation, potentially compromising experimental accuracy.
In this issue of Cell Culture Academy, we use rat pulmonary great artery endothelial cells as an example to systematically outline key isolation steps, providing a standardized and efficient approach to support robust downstream research.
Ⅰ. Basic Information
Cell Name: Rat pulmonary great artery endothelial cells
Tissue Source: Pulmonary great artery
Cell Description: The pulmonary great artery (pulmonary trunk) is a short, thick vessel located within the pericardium that arises from the right ventricular outflow tract and courses anterior to the aortic arch. Inferior to the arch, it bifurcates into the left and right pulmonary arteries, which enter the lungs via the pulmonary hilum and deliver deoxygenated blood for oxygenation. Pulmonary great artery endothelial cells form the inner lining of this vessel and play essential roles in maintaining vascular homeostasis through cytokine and mediator secretion, as well as regulation of coagulation and fibrinolysis.
Morphological characteristics: Upon adherence, these cells exhibit a monolayer, polygonal “cobblestone-like” morphology with well-defined borders and uniform appearance.
Ⅱ. Operational Procedures
1.Dissection Protocol
Wistar, Sprague-dawley (SD), or other rat strains aged 20-30 days were used. Animals were euthanized by overdose of sodium pentobarbital, disinfected, and placed in the supine position.
The chest skin was incised to expose the sternum, the thoracic cavity was opened, and the thymus was removed.
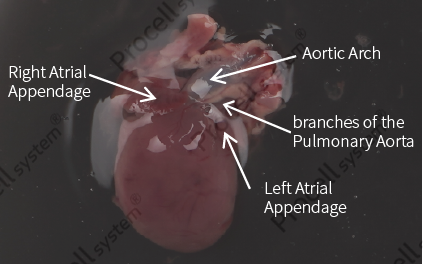
The atrium was gently elevated to expose the pulmonary great artery and aortic arch. Tissue containing the pulmonary great artery, including the heart, aortic arch, and a small amount of lung tissue, was excised slightly left of the vessel apex (Figure 1).
2.Tissue Processing
Rinse the tissue thoroughly with a specialized cleaning solution to remove blood from rat pulmonary great artery endothelial cells.
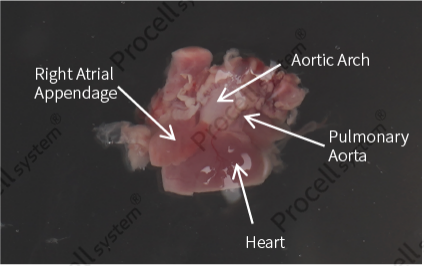
Excise the lower half of the heart, gently squeeze the vessels to remove clotted blood, and rinse again (Figure 2).
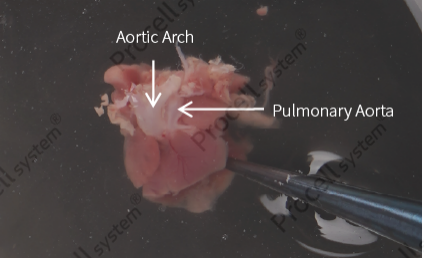
Remove fat between the aortic arch and pulmonary great artery, identify the branch vessels of the pulmonary artery (Figure 3), and dissect them.
Cut at the vessel root connecting to the heart to isolate the aortic arch and pulmonary artery tissue (Figure 4), and further remove any residual adipose tissue.
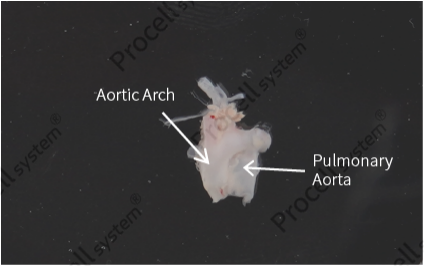
Open the aortic arch and pulmonary great artery (Figure 5), retain only the pulmonary artery tissue, and longitudinally section it (Figure 6).
3.Tissue Digestion
The processed pulmonary great artery tissue was incubated in a proprietary digestion solution at 37 ℃ with 5% CO2 for 30 min.
After digestion, a dedicated washing solution was added, and the tissue was gently pipetted approximately 30 times to release adherent endothelial cells into suspension. The resulting cell suspension was then collected.
4.Cell Isolation
The collected suspension was filtered through a 100-μm cell strainer. The filtrate was transferred to a centrifuge tube, centrifuged at 1,200 rpm for 5 min, the supernatant was discarded, and the pellet was retained.
5.Cell Culture
Coating of culture vessels: Add 1 mL of the plating solution for rat pulmonary great artery endothelial cells to a 6-cm culture dish and incubate at 37 ℃ with 5% CO2 for 0.5-2 h.
Cell seeding: Resuspend cells in Rat Pulmonary Great Artery Endothelial Cell Complete Medium, seed into the coated vessels, and culture at 37 ℃ with 5% CO2.
Medium change and subculture: Replace medium for the first time after 48 h, then every 2-3 days. Initiate subculture when cells reach 80-90% confluence.
For detailed instructions, refer to the Rat Pulmonary Great Artery Endothelial Cell Isolation and Culture Kit manual.
Ⅲ. Cell Identification
1.Morphological Identification
The cells display a “cobblestone-like” morphology, characteristic of endothelial cells.
2. Marker Identification
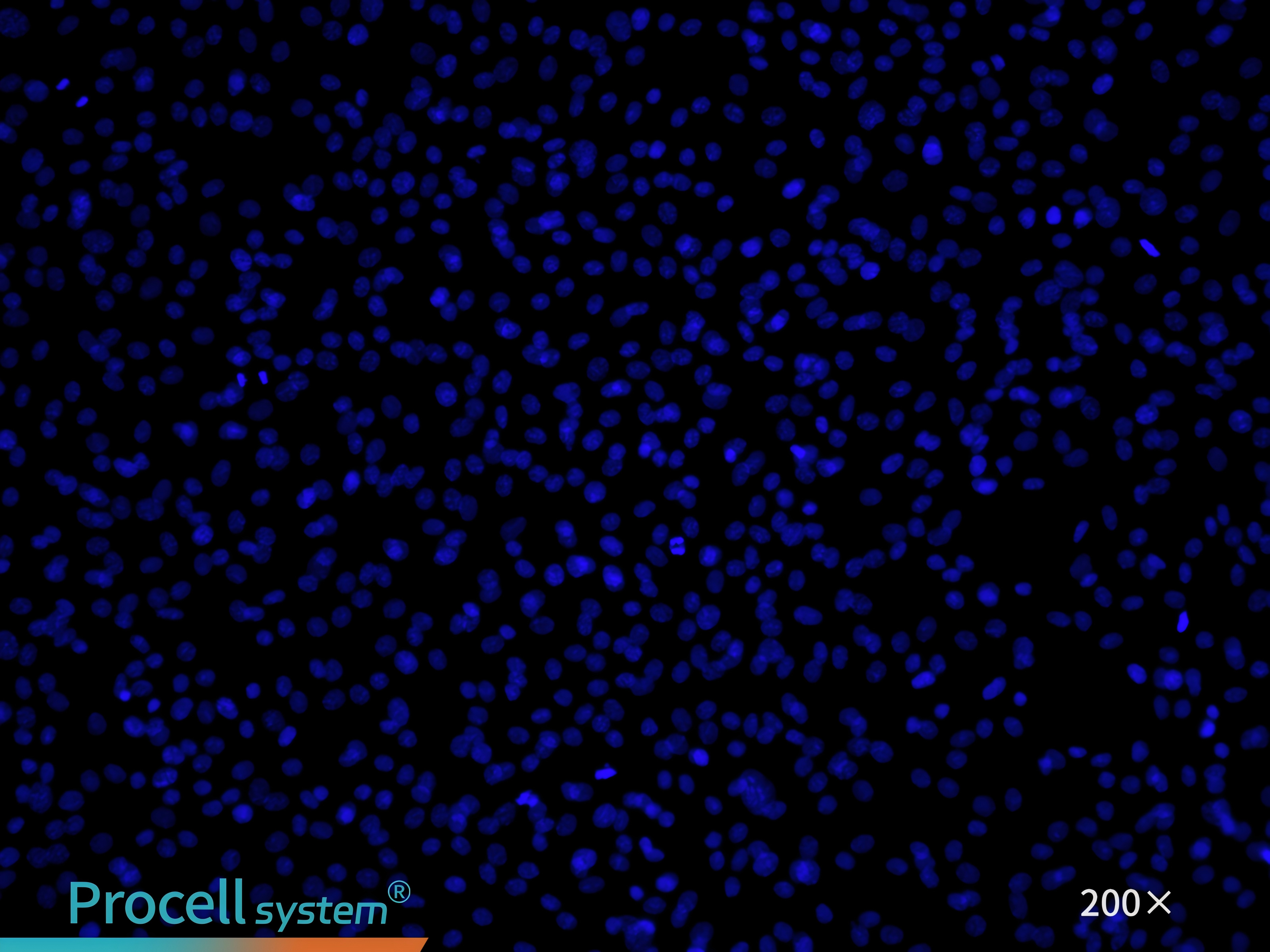
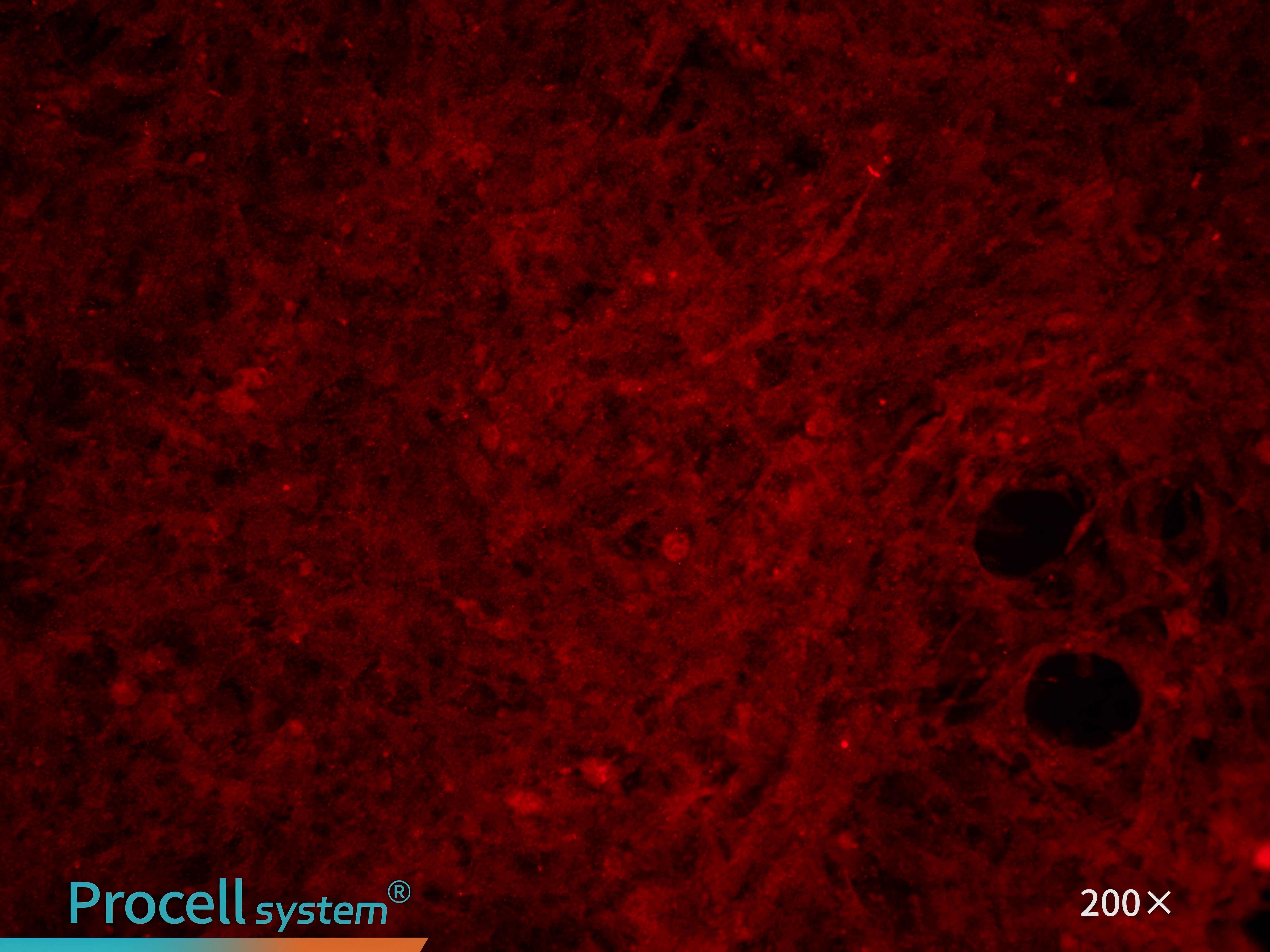
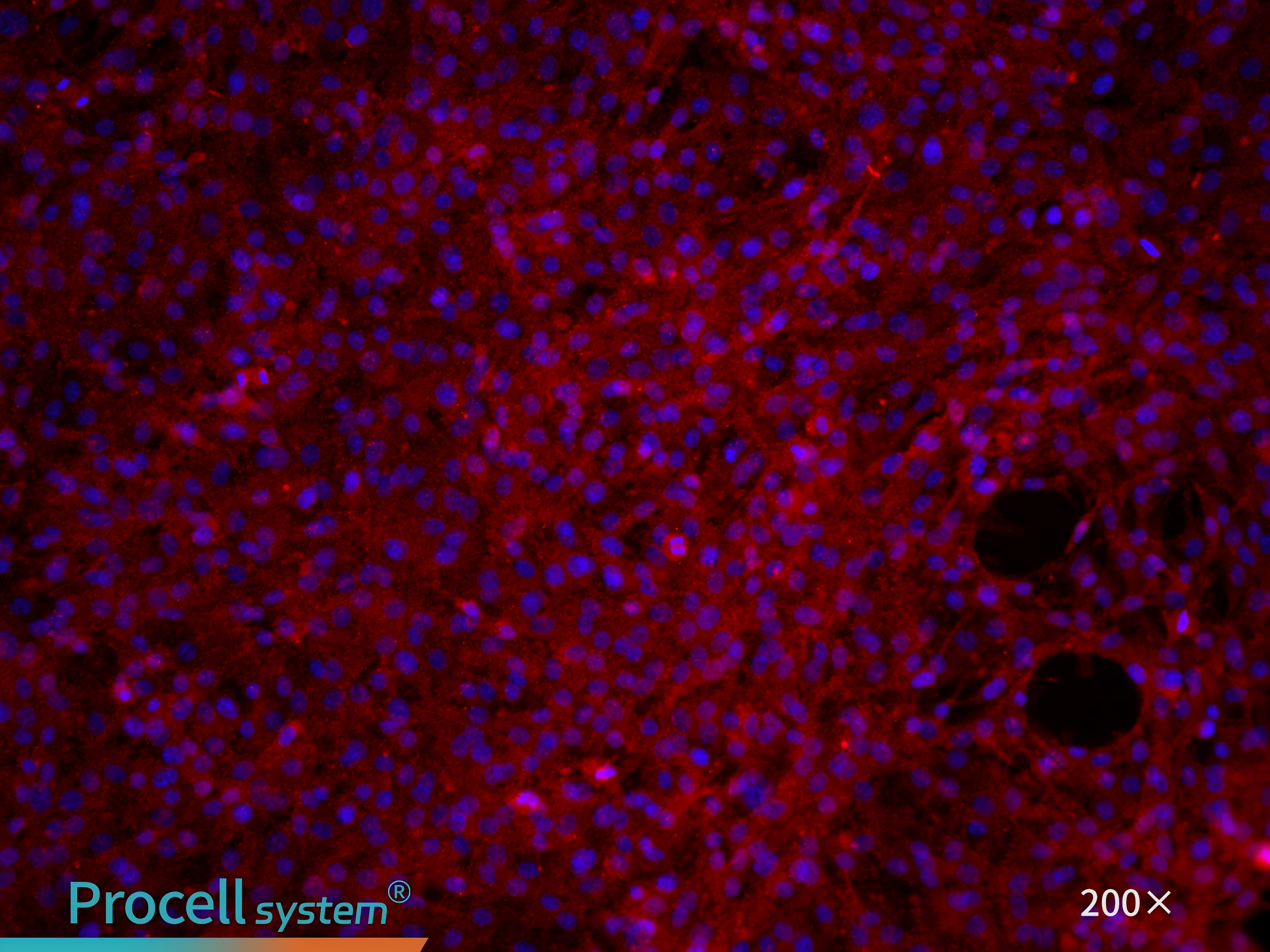
CD31 immunofluorescence staining confirms that the cell purity is greater than 90% (Figure 7).
Figure 7. Immunofluorescence identification of rat pulmonary great artery endothelial cells
IV. Culture Conditions
Coating: Poly-L-Lysine Solution (0.1 mg/mL) or Gelatin Coating Solution (0.1%)
Culture medium: Rat Pulmonary Great Artery Endothelial Cell Complete Medium
Medium change frequency: Every 2-3 days
Growth characteristics: Adherent cells
Cell morphology: Typical endothelial-like appearance
Passage ratio: 1:2
Passage characteristics: Can be passaged 2-3 times
Digestion solution: 0.25% Trypsin Solution
V. Application Directions
In pulmonary vascular disease research, pulmonary great artery endothelial cells are widely used to study imbalances in cell proliferation and apoptosis, mitochondrial dynamics, endothelial-mesenchymal transition, inflammatory responses, extracellular vesicle-mediated intercellular communication, and functional validation of genes or non-coding RNAs.
Example 1: In a hypoxia model of human pulmonary great artery endothelial cells, Hpgd (hydroxyprostaglandin dehydrogenase) expression decreases over varying hypoxia durations. Hpgd regulates vascular remodeling by modulating proliferation, apoptosis, adhesion, and angiogenic capacity in hypoxia-treated cells and contributes to the development of hypoxic pulmonary hypertension[1].
Example 2: A hypoxia model using mouse pulmonary great artery endothelial cells was employed to investigate the role of circKrt4 in hypoxic pulmonary hypertension. CircKrt4 promotes disease progression through a dual mechanism: it binds Pura, driving endothelial-mesenchymal transition and upregulating N-cadherin, while simultaneously inhibiting Glpk mitochondrial transport, leading to mitochondrial dysfunction. Through coordinated regulation of Pura and Glpk, circKrt4 exacerbates endothelial cell injury and accelerates disease progression[2].
References
1.He M, Tao K, Xiang M, Sun J. Hpgd affects the progression of hypoxic pulmonary hypertension by regulating vascular remodeling. BMC Pulmonary Medicine. 2023 Apr 13; 23 (1): 116.
2.Qingjiao Chen, Mingui Chen, Jizhen Wang. Prognostic genes related to mitochondrial dynamics and mitophagy in diffuse large B-cell lymphoma are identified and validated using an integrated analysis of bulk and single-cell RNA sequencing, Frontiers in Immunology, 2025 (16).
3.Ma C, Wang X, Zhang L. Super enhancer-associated circular RNA-circKrt4 regulates hypoxic pulmonary artery endothelial cell dysfunction in mice. Arteriosclerosis Thrombosis And Vascular Biology. 2023;43(7):1179-1198.
Prev: A No-mistake Guide to High-activity Rat Articular Chondrocyte Isolation
Next: Optimized Protocols for Hepatic Parenchymal Cell Isolation, Culture, and Model Development