Unlocking the Hidden Potential of Mesenchymal Stem Cells: From Characterization to In Vitro Culture
Oct 24,2025
Mesenchymal stem cells (MSCs) are adult stem cells. With their unique biological characteristics and remarkable therapeutic potential, MSCs have become a rising star in the field of disease research. As of May 2024, there were more than 1,200 ongoing clinical trials involving MSCs worldwide, and over 27 MSC-related products had been approved, providing preliminary evidence of their clinical value.
To help readers quickly and systematically grasp the essential knowledge of MSCs, we have planned a series of posts covering the following topics in sequence: an overview of MSCs, in vitro culture and expansion, trilineage differentiation, and applications in cell therapy.
In this issue of Cell Culture Academy, we will provide a comprehensive overview of MSCs, including their key characteristics, biological functions, research applications, and in vitro culture strategies, enabling you to quickly become familiar with this cutting-edge area of study.
I. Basic Characteristics of MSCs
Mesenchymal stem cells (MSCs), originating from the mesoderm, exhibit robust self-renewal and multilineage differentiation potential. Their defining features include self-renewal, low immunogenicity, high viability, chemotactic responsiveness, and non-tumorigenicity, which have attracted significant attention in biomedical research.
The International Society for Cell & Gene Therapy (ISCT) has established minimal standards for identifying Mesenchymal Stem Cells (MSCs).
1.Adherent: MSCs must be plastic-adherent when maintained in standard culture conditions.
2.Surface markers: MSCs must express CD105, CD73, and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR.
3.Differentiation potential: MSCs must be capable of differentiating into osteoblasts, adipocytes, and chondroblasts in vitro.
Note: As MSCs research advances, the ISCT identification criteria may be refined or updated.
MSCs can be isolated from multiple sources, including bone marrow, umbilical cord, adipose tissue, teeth, and placenta, and have the capacity to differentiate into various cell types (Figure 1).
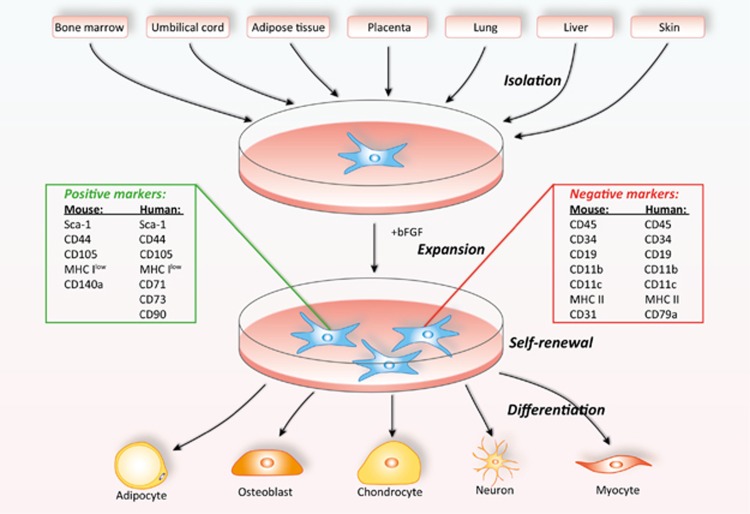
Figure 1. Tissue sources and differentiation potential of MSCs (Reproduced from reference[1])
MSCs derived from different sources exhibit notable variations in cell yield, proliferative capacity, and functional properties. These differences play a critical role in guiding their selection for both research and clinical applications. The key characteristics of MSCs from commonly used sources are summarized in Table 1.
Table 1. Characteristic analysis of MSCs from common sources
| Cell Types | Tissue Origin | Key Features |
| BM-MSCs | Bone marrow aspirate (invasive procedure) | Longest history of research; stable differentiation potential; well-characterized immunomodulatory mechanisms; limited cell yield; age-dependent proliferation and differentiation capacity |
| UC-MSCs | Neonatal umbilical cord (postnatal discard) | Abundant source; high proliferative capacity; low immunogenicity; suitable for allogeneic applications |
| AD-MSCs | Adipose tissue (obtained via liposuction) | High cell yield; easily accessible; strong proliferative capacity; differentiation potential slightly lower than BM-MSCs |
| SF-MSCs | Joint synovial fluid (arthrocentesis) | Low cell yield; moderate differentiation potential; exhibits immunomodulatory activity |
| P-MSCs | Placental tissue (postnatal discard) | Abundant source; strong immunomodulatory capacity; high proliferative potential |
| DP-MSCs | Dental pulp | Limited cell yield; source restricted by tooth availability; good proliferative capacity; notable neurogenic differentiation potential |
II. Core Functions of MSCs
The therapeutic potential of MSCs derives largely from their four fundamental biological functions, which work synergistically to support tissue regeneration and enhance clinical applications.
1. Self-Renewal and Proliferative Capacity
MSCs demonstrate strong self-renewal and proliferative capabilities, allowing them to continuously divide, generate new stem cells, and preserve both their population stability and functional integrity.
2. Multilineage Differentiation Potential
MSCs possess the capacity to differentiate into multiple cell lineages, including osteoblasts, chondrocytes, adipocytes, cardiomyocytes, and neural cells, among others.
3. Tissue Repair and Regeneration
MSCs secrete a wide range of growth factors and immunomodulatory cytokines, as well as establish a supportive microenvironment that promotes cellular recovery and tissue remodeling.
4. Immunomodulatory Function
MSCs exert potent immunomodulatory effects by regulating immune cell activity, attenuating excessive inflammatory responses, and contributing to immune tolerance.
III. Research and Applications of MSCs
Owing to their multifunctional properties, MSCs have been extensively studied in a variety of preclinical models and clinical trials targeting a broad spectrum of diseases[2-4]. Their therapeutic applications span autoimmune and inflammatory disorders, neurodegenerative diseases, orthopedic injuries, and other pathological conditions (Figure 2).
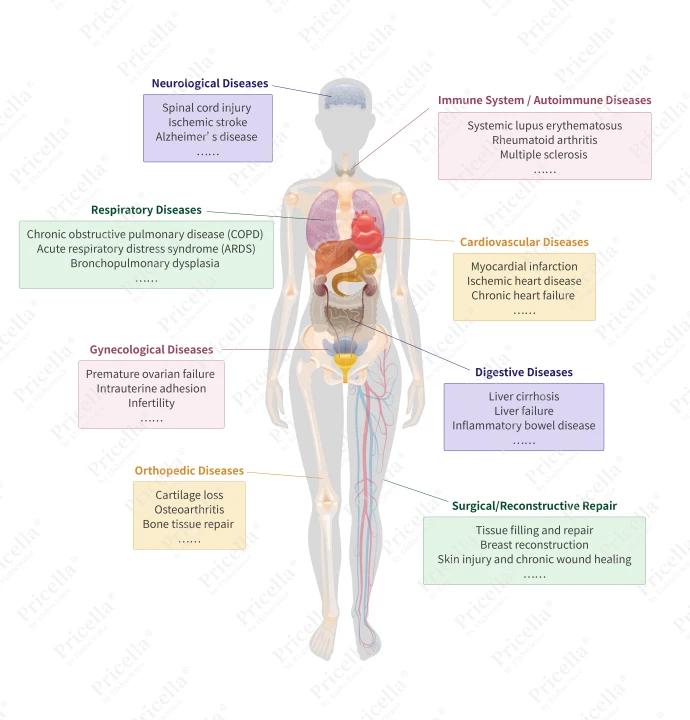
Figure 2. Therapeutic applications of MSCs in various diseases
MSCs derived from different tissue sources exhibit distinct biological characteristics, immunomodulatory capacities, and differentiation potentials. These variations affect their suitability and potential advantages in treating specific diseases. Selecting the most appropriate MSC source for a given pathological condition remains an active area of research[2-3] (Table 2).
Table 2. Representative Application of MSCs from Major Tissue Sources in Disease Treatment
| Cell Types | Therapeutic Applications | Key Features |
| BM-MSCs | Osteoarthritis, spinal cord injury, graft-versus-host disease (GVHD), amyotrophic lateral sclerosis (ALS), cardiovascular disorders, endocrine dysfunction, assisted reproductive technologies | Well-characterized immunomodulatory mechanisms; extensive long-term safety data |
| UC-MSCs | Myocardial infarction, liver fibrosis, neurodegenerative diseases, respiratory disorders | Low immunogenicity enabling allogeneic transplantation, age-independent and readily available source |
| AD-MSCs | Plastic and reconstructive surgery (tissue augmentation and repair, breast reconstruction), arthritis and cartilage repair, wound healing, autoimmune disease treatment, cardiovascular disorders | High cell yield; strong adipogenic and fibroblastic differentiation potential; ideal for soft tissue regeneration |
IV. In Vitro Culture Strategies for MSCs
With the growing demand for stem cell-based therapies, the in vitro expansion of MSCs has become increasingly critical. Choosing an appropriate culture system and methodology is essential not only for preserving MSC characteristics but also for maximizing their translational and clinical potential.
1. Culture Systems
The most commonly used culture systems include serum-containing media, serum-free media, and media supplemented with serum alternatives (Table 3).
Table 3. Comparative Analysis of In Vitro Culture Systems for MSCs
| Culture System | Advantages | Limitations | Typical Applications |
| Cell Complete Medium | Low cost; widely accessible in laboratories; high cellular adaptability | High batch-to-batch variability; potential clinical risks | Basic research; routine MSC expansion |
| Serum-free Medium | Chemically defined; free of serum and animal-derived components; minimal batch variability; enables high-purity exosome production | Relatively expensive; some MSC types exhibit poor adaptability | Exosome research; drug development |
| Serum-free Medium, with Serum Replacement | Provides serum-mimicking nutrients; reduces serum-related risks; cost is relatively moderate. | / | General research; preclinical studies |
2.Culture Methods
MSCs can be cultured using either two-dimensional (2D, traditional) or three-dimensional (3D) approaches.
In 2D culture, cells adhere to and proliferate on the flat surface of culture vessels. This method is straightforward, cost-effective, and remains widely used in regenerative medicine and basic stem cell research.
However, 2D culture differs substantially from the natural 3D microenvironment found in vivo. MSCs grown on flat surfaces are prone to morphological changes, altered proliferation kinetics, and reduced differentiation potential. Prolonged 2D culture can also lead to the loss of stemness, premature cellular senescence, and diminished genomic stability.
To address these limitations, 3D culture technologies have been developed. 3D culture provides a microenvironment that more closely mimics in vivo conditions, using either scaffold-free spheroids or scaffold-based systems such as hydrogels (e.g., alginate, collagen, Matrigel). This setup allows MSCs to grow, proliferate, and migrate within a spatially organized structure, preserving their native morphology and functional characteristics.
Although 3D culture is more technically demanding, costly, and harder to standardize, it offers substantial advantages in replicating in vivo physiology and maintaining MSC biological properties. The choice of culture method should be guided by the specific objectives of the experiment.
References
[1]Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?. Cell Death & Differentiation. 2016; 23 (7): 1128-1139.
[2]Zhidu S, Ying T, Rui J, et al. Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities. Stem Cell Research & Therapy. 15, 266 (2024).
[3]Zhou J, Shi Y, et al. Mesenchymal stem/stromal cells (MSCs): origin, immune regulation, and clinical applications. Cellular & Molecular Immunology. 20, 555–557 (2023).
[4]Han X, Liao R, Li X, et al. Mesenchymal stem cells in treating human diseases: molecular mechanisms and clinical studies. Signal Transduction and Targeted Therapy. 10, 262 (2025).
[5]Li J, Wu Z, Zhao L, et al. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Research & Therapy. 2023; 14 (1): 381.
[6]Moldaschl J, Chariyev-Prinz F, Toegel S, et al. Spheroid trilineage differentiation model of primary mesenchymal stem/stromal cells under hypoxia and serum-free culture conditions. Frontiers in Bioengineering and Biotechnology. 2024; 12: 1444363.
Bicer M, Cottrell GS, Widera D. Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Research & Therapy. 2021; 12 (1): 31.
Prev: Unveiling the Key Points of MDA-MB-231 Culture: Making Breast Cancer Research Easier
Next: Unlocking the Hidden Potential of Mesenchymal Stem Cells: Efficient Culture and Stable Expansion


