Unlocking the Hidden Potential of Mesenchymal Stem Cells: Efficient Culture and Stable Expansion
Oct 27,2025
Mesenchymal stem cells (MSCs) are adult stem cells. With their unique biological characteristics and remarkable therapeutic potential, MSCs have become a rising star in the field of disease research. As of May 2024, there were more than 1,200 ongoing clinical trials involving MSCs worldwide, and over 27 MSC-related products had been approved, providing preliminary evidence of their clinical value.
To help readers quickly and systematically grasp the essential knowledge of MSCs, we have planned a series of posts covering the following topics in sequence: an overview of MSCs, in vitro culture and expansion, trilineage differentiation, and applications in cell therapy.
This issue of Cell Culture Academy explores strategies for the efficient and stable in vitro culture and expansion of MSCs.
I. MSC Culture Key Challenges
MSCs are adherent adult stem cells that are highly sensitive to their microenvironment. During in vitro culture, several key challenges are frequently encountered:
1. Limited proliferative capacity and cellular senescence
With successive subculturing, MSCs progressively accumulate DNA damage and experience telomere shortening, ultimately leading to cellular senescence and reduced proliferative potential.
2.Loss of stemness
As the passage number increases, MSCs tend to lose their multilineage differentiation capacity and self-renewal ability. Therefore, careful control and monitoring of passage number are essential to preserve their biological characteristics and functional stability.
3.Pronounced cellular heterogeneity
In addition to senescence and the decline in stemness during extended culture, MSCs derived from different tissue sources (such as bone marrow, adipose tissue, and umbilical cord) display distinct proliferation rates, adhesion properties, and differentiation potentials. Therefore, culture conditions must be optimized according to the specific cell source.
Ⅱ. MSC Culture Protocol
A well-defined culture system is a key determinant of the successful in vitro expansion of MSCs. The system should not only support robust proliferation while also preserve stemness and functional integrity. Currently, MSC culture methods are primarily classified into serum-containing and serum-free systems.
1. Serum-Containing Systems
The serum-containing culture system typically consists of a basal medium, fetal bovine serum (FBS), and essential cytokines. This system remains the most commonly used approach in laboratory settings.
A.Basal medium
Commonly used basal media include α-MEM, high-glucose DMEM, and low-glucose DMEM. These formulations provide essential nutrients such as amino acids, carbohydrates, and minerals required for MSC growth.
B.FBS
FBS provides a complex mixture of cytokines, hormones, and adhesion molecules that promote MSC attachment and proliferation. Pricella® offers Fetal Bovine Serum for Mesenchymal Stem Cells.
C.Cytokines
During prolonged in vitro subculture, MSCs may experience proliferation arrest. Supplementation with cytokines can activate relevant signaling pathways to enhance cell growth. Commonly used cytokines include FGF-2, EGF, and PDGF.
Pricella® provides a series of Marrow Mesenchymal Stem Cell Complete Medium containing all essential components for cell growth, ready to use without further supplementation.
2. Serum-Free Culture System
Serum-free culture media feature well-defined compositions, high consistency, and minimal batch-to-batch variation. The absence of animal-derived components reduces biological risk and experimental variability, making these media suitable for culturing human MSCs derived from multiple tissue sources.
Pricella® offers serum-free MSC media tailored for specific experimental needs:
Human Mesenchymal Stem Cells Serum-Free Medium (Product No.: CM-SC01)
Human Mesenchymal Stem Cells Serum-Free Medium, with Serum Replacement (Product No.: CM-SC02)
3. Auxiliary Reagents
The appropriate selection and use of auxiliary reagents can significantly enhance MSC viability, adhesion, and the maintenance of stemness during in vitro culture.
A.Antibiotics
Antibiotics, such as penicillin–streptomycin, are commonly used to prevent bacterial or fungal contamination. However, prolonged use may mask low-level contamination and potentially alter cellular function; therefore, they should be used judiciously.
B.Coating Reagents
Coating culture surfaces with extracellular matrix components such as collagen, fibronectin, or gelatin can improve MSC adhesion, particularly in primary or low-density cultures. Under serum-free or low-serum conditions, coating reagents play a critical role in enhancing cell survival and culture stability.
The choice of coating material should be optimized based on the culture medium type, experimental objectives, and downstream applications (e.g., maintenance of stemness or differentiation assays).
C.Dissociation Reagents
During MSC passaging, gentle and efficient dissociation reagents, such as Recombinant Trypsin Solution or Accutase Cell Detachment Solution, should be employed to preserve cell viability, surface markers, and stemness properties.
For serum-free or animal-free systems, reagents clearly labeled as animal component-free should be used to ensure the reliability of subsequent MSC applications.
Ⅲ. MSC Subculture Practices
Given the sensitivity of MSCs and their limitations in in vitro expansion, strict control of key culture parameters is essential to maintain cell quality and functionality:
1.Control Passage Number
MSCs exhibit a finite proliferative capacity. Prolonged passaging can lead to reduced proliferation, diminished osteogenic differentiation potential, and altered cellular characteristics. Regular monitoring of cell morphology, adhesion, and growth rate is recommended to assess cell health, and any deviations should be addressed promptly.
2.Optimize Seeding Density
Suboptimal seeding density can impair cell adhesion (if too low) or trigger premature differentiation (if too high). Subculture is generally performed when cells reach 70-80% confluence to maintain optimal growth conditions.
3.Regularly Refresh Culture Medium
MSCs are metabolically active, and the accumulation of waste metabolites can alter medium pH and compromise cell function. Replacing the culture medium every 2-3 days helps maintain a stable microenvironment.
4.Refine the Digestion Procedure
Careful selection of digestive enzymes and precise control of digestion time are critical. Avoid excessive pipetting or prolonged enzyme exposure, which can damage cell membranes and degrade surface markers, potentially affecting downstream analyses.
IV. MSCs Identification Methods
How can we verify that cultured cells are truly MSCs?
The International Society for Cellular Therapy (ISCT) established the gold standard criteria for MSC identification in 2006.
1.Morphological Assessment
MSCs typically display a fibroblast-like morphology, appearing elongated and spindle-shaped under an inverted microscope.
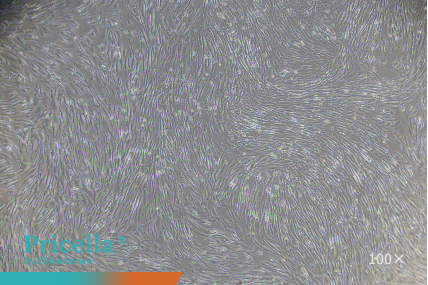
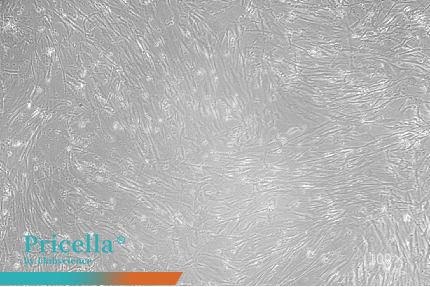
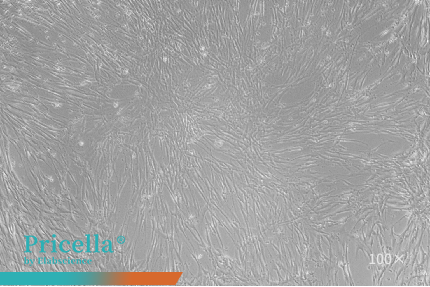
Human umbilical cord MSCs and human adipose-derived MSCs maintain stable proliferation and retain their characteristic morphology during serial subculturing in a Human Mesenchymal Stem Cells Serum-Free Medium (Figure 1).
Figure 1. Observation of MSCs at different passages
2.Detection of Surface Markers
Surface marker profiling serves as a fundamental criterion for the identification of MSCs.
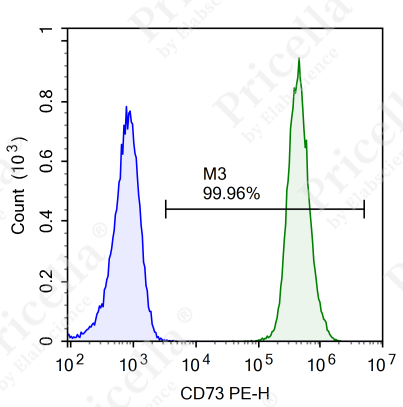
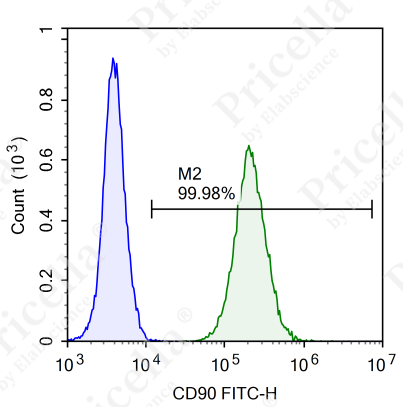
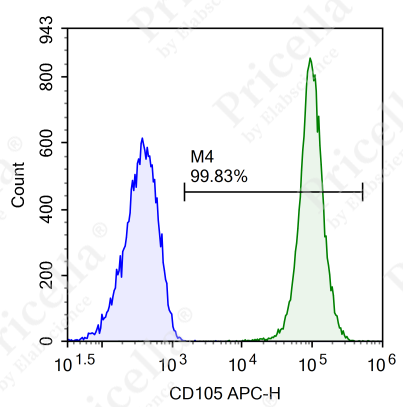
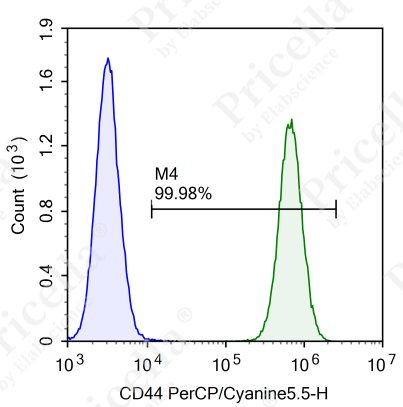
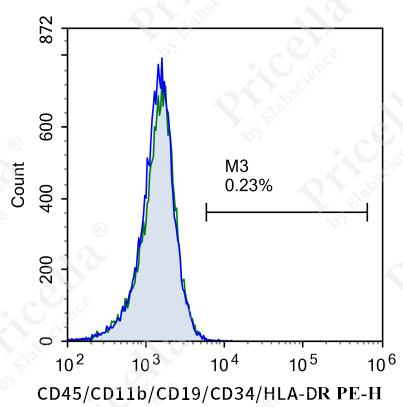
For instance, in human umbilical cord MSCs, flow cytometry is employed to assess the expression of specific markers (Figure 2).
Positive markers (expression ≥ 95%): CD73, CD90, CD105, CD44.
Negative markers (expression ≤ 2%): CD45, CD11b, CD19, CD34, and HLA-DR.
Figure 2. Flow cytometry analysis of surface markers in human umbilical cord MSCs
Note: Surface marker expression may vary slightly across MSCs from different tissue sources.
3.Verification of Differentiation Potential
Osteogenic, adipogenic, and chondrogenic differentiation are considered the “gold standards” for MSC identification.
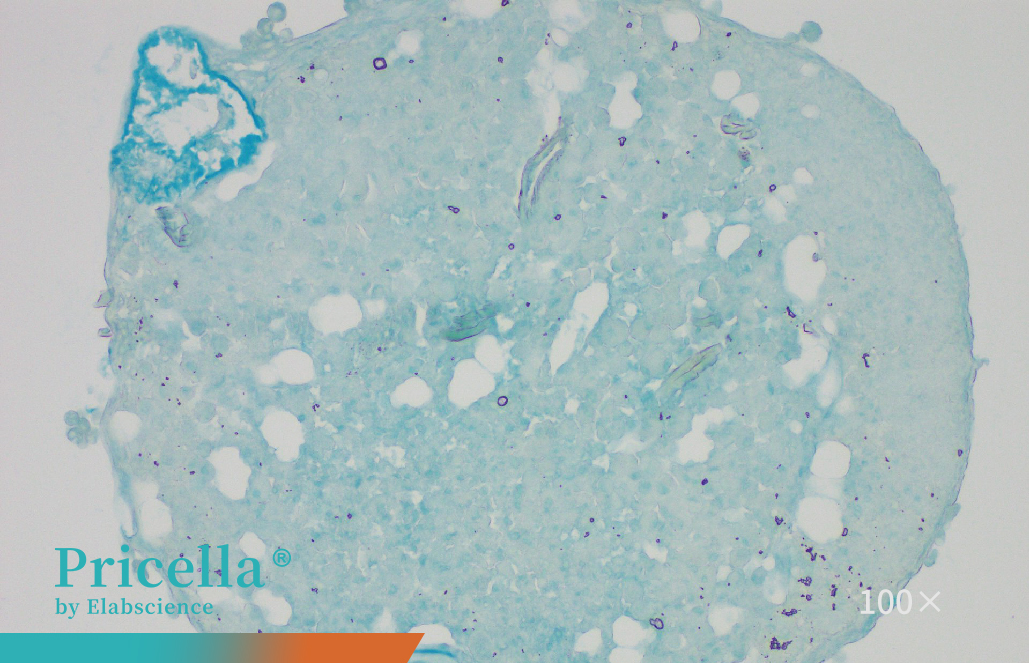
Rat bone marrow MSCs were cultured in differentiation media for osteogenesis, adipogenesis, and chondrogenesis, and successfully differentiated into osteoblasts, adipocytes, and chondrocytes, respectively. Differentiation was confirmed using Alizarin Red, Oil Red O, and Alcian Blue staining (Figure 3).
Figure 3. Trilineage differentiation of rat bone marrow MSCs
MSC culture is a technically demanding process that requires both scientific knowledge and practical experience. Owing to their adherent nature, sensitivity to the culture environment, and limited proliferative capacity, MSCs are generally considered challenging to maintain. Nevertheless, careful selection of culture media, strategic use of supportive reagents, and optimization of procedural techniques can substantially reduce failure rates and enable the consistent production of high-quality cells.
Prev: Unlocking the Hidden Potential of Mesenchymal Stem Cells: From Characterization to In Vitro Culture
Next: Accutase Cell Detachment Solution: Gentle on Cells, Powerful in Performance