A Practical Guide to Isolation and Culture of Rat Cortical Neurons
Dec 02,2025
Primary cells are defined as cells directly isolated from tissues or organs without prior subculture. At the initial stages of in vitro culture, they retain the key physiological properties of their native tissues, thereby providing an accurate representation of the in vivo microenvironment. As a result, primary cells are considered robust models for molecular biology and pharmacological studies. Primary cortical neurons enable systematic investigation of neuronal growth, differentiation, and functional dynamics under controlled culture conditions. This approach offers reliable experimental evidence to support research on neurological disease mechanisms, prevention, and therapeutic development.
It should be noted that isolation methods differ among primary cell types and typically require a high level of technical proficiency. In this issue of the Cell Culture Academy, we provide a systematic overview of the isolation and culture procedures for rat cortical neurons to support the smooth advancement of your neuroscience research.
Ⅰ. Basic Information
Cell Name: Rat Cortical Neurons
Tissue Origin: Cerebral Cortex
Cell Description: Cortical neurons are the fundamental structural and functional units of the central nervous system. Each neuron consists of a soma and projections. The soma is located in the brain, spinal cord, or ganglia, while neuronal projections extend throughout various organs and tissues. These projections, which are extensions of the soma, are classified as dendrites and axons. Neurons possess one or more dendrites that receive stimuli and transmit signals to the soma, whereas a single axon conducts impulses from the soma to other neurons or effector tissues such as muscles or glands.
Morphological Characteristics: Following substrate adhesion, neurons initially develop sparse projections that progressively increase in number. These projections elongate and form interconnected networks as the soma enlarges and a distinct peripheral halo appears. Peak morphological integrity is typically observed on days 10-12 of culture. Prolonged culture results in characteristic degeneration, including reduced projections, loss of defined cell boundaries, disappearance of the halo, and eventual progression toward apoptosis or degenerative states.
Ⅱ. Operational Procedures
1.Dissection Protocol
Select 1-2-day-old Wistar, Sprague-Dawley (SD), or other rat strains. Following euthanasia by decapitation, rapidly remove the intact brain under sterile conditions.
2.Tissue Processing
A. Cerebral Cortex Isolation
Stabilize the brain tissue. Using micro-curved forceps, carefully separate the left and right cerebral hemispheres from the cerebellum by clamping along the regions indicated by the three solid black lines (Figure 1).
Invert both hemispheres, remove the cerebral white matter, and retain the cerebral cortex (Figure 2).
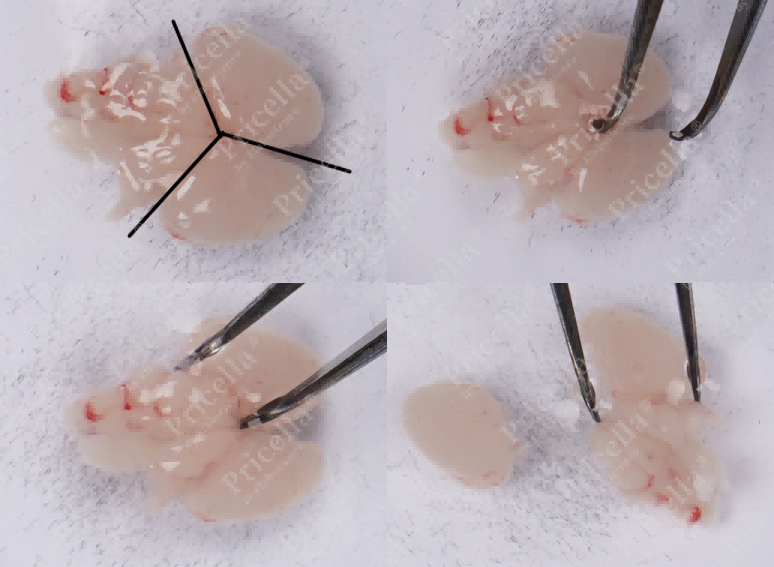
Figure 1. Separate cerebellum and brain
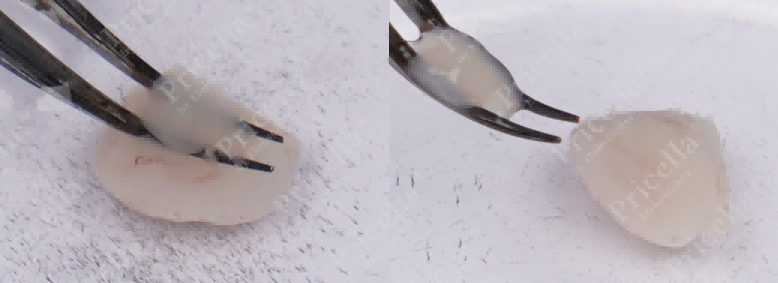
Figure 2. Separate the essence and retain the cerebral cortex
B.Meninges Removal (Two approaches)
Option 1: Flip the left and right hemispheres to expose the meninges. Gently stabilize the tissue and peel off the meninges by pulling in the opposite direction (Figure 3), leaving pure cortical tissue.
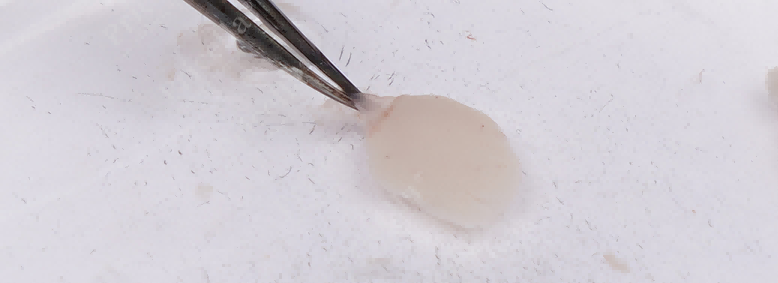
Figure 3. Stabilize the tissue with the left hand, and gently pull to strip off the meninges
Option 2: Line a culture dish with filter paper and roll the cortical tissue 1-2 times on it. The red meninges separate from the cortical tissue, leaving clean cortical tissue behind.
C.Tissue Mincing
Mince the meninges-free cortical tissue into 1 mm³ pieces. Transfer the tissue to a centrifuge tube, centrifuge, discard the supernatant, and collect the pellet.
3.Tissue Digestion
Add the specialized digestive solution to the pellet and incubate until the tissue is fully dissociated. After digestion, filter the suspension, wash thoroughly, and resuspend to obtain a single-cell suspension.
4.Cell Culture
Resuspend the cell pellet in Rat Cortical Neuron Complete Medium. Seed cells into a T25 flask pre-coated with planting solution, and add screening solution. Incubate statically at 37°C with 5% CO2 in a humidified incubator.
Perform the first complete medium change 48 h after seeding, then replace the medium every 2-3 days thereafter.
For detailed procedures, refer to the official Rat Cortical Neuron Cell Isolation and Culture Kit instruction manual.
Ⅲ. Cell identification
1. Morphological Identification
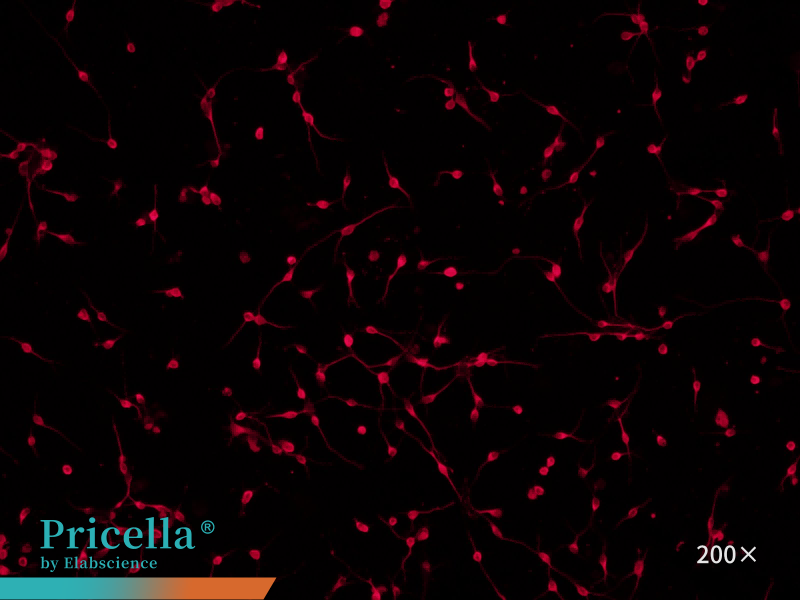
Under optical microscopy, cells display strong adhesion and typical neuron-like morphology, appearing polygonal or irregular in shape.
2. Marker Identification
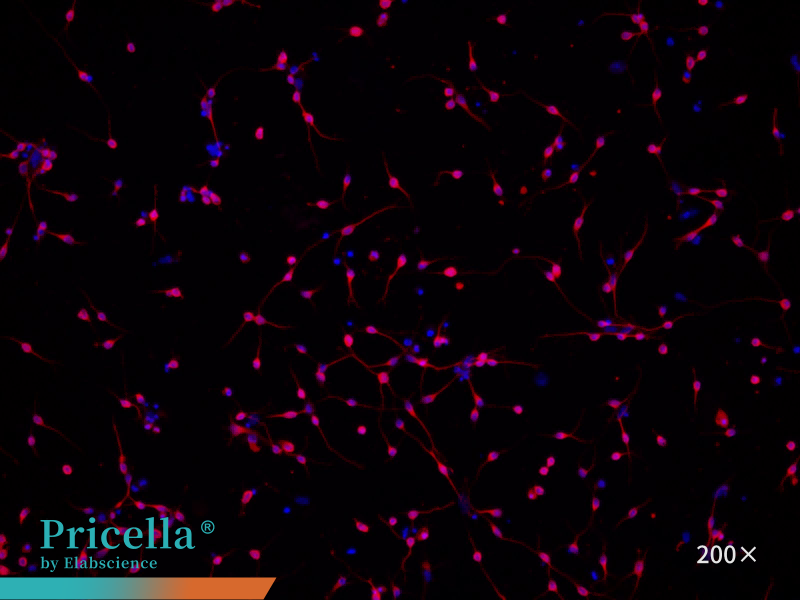
Immunofluorescence staining with β-Tubulin-III as the primary antibody demonstrates > 90% cell purity under fluorescence microscopy (Figure 4).
Figure 4. Immunofluorescence identification of rat cortical neurons
IV. Culture Conditions
Coating: Poly-L-lysine solution (1 ×) or Poly-L-Lysine Solution (10 ×) diluted to 0.1 mg/mL
Coating Medium: Rat Cortical Neuron Complete Medium (containing B-27 Supplement, Penicillin, Streptomycin, etc.)
Medium Change Frequency: Every 2-3 days
Growth Characteristics: Adherent
Morphology: Neuron-like
Passage Ratio: Not applicable (non-passaged)
Passage Characteristics: Terminally differentiated, non-proliferating cells
Digestion Solution: 0.125% Trypsin solution
V. Application Directions
Cortical neurons, due to their structural and functional similarity to the human nervous system, are widely used in neuroscience research. Key application areas include:
1.Neurodegenerative Disease Research
In vitro neuronal injury models simulate pathological features of diseases such as Alzheimer's and Parkinson's, including β-amyloid accumulation, α-synuclein abnormalities, and mitochondrial dysfunction, to investigate cellular mechanisms underlying neurodegeneration.
2. Drug Screening and Efficacy Evaluation
Pathological cortical neuron models are used to screen candidate compounds that enhance neuronal survival, improve synaptic function, or restore neural networks. Assessments include effective concentration determination and mechanism analysis using metrics such as cell viability, apoptosis markers, synaptic density, and electrophysiological activity.
3. Neural Development and Synaptic Plasticity
In vitro observation of cortical neuron development, including dendritic extension, axonal growth, synapse formation, and maturation, enables study of key genes or proteins regulating neural development and synaptic plasticity. Analyses combine morphological evaluation, molecular marker detection, and electrophysiological testing.
4. Neurotoxicity Assessment
Primary cortical neurons’ high sensitivity to environmental toxins, drug side effects, or heavy metals allows evaluation of neurotoxicity. Common indicators include cell viability, apoptosis, synaptic damage, and membrane potential alterations, providing experimental support for safety assessments.
5. Gene Function Studies
Manipulation of gene expression through overexpression, knockdown, or CRISPR editing allows assessment of cortical neuron morphology, function, and survival, clarifying gene roles in neural development, survival, and disease pathogenesis. Verification integrates molecular biology and electrophysiological methods.
Prev: Analysis of Cell Culture Medium: Composition, Preparation, and Troubleshooting
Next: Breaking the Transfection Barrier in A549 Cells: Essential Techniques for Robust Gene Expression