Your Complete Guide to Isolating Bone Marrow Monocytes
Dec 23,2025
In immunology, hematopoiesis, inflammation regulation, and regenerative medicine, bone marrow monocytes are essential experimental tools. They play a pivotal role in studies on monocyte/macrophage differentiation, the hematopoietic microenvironment, tumor immunology, and tissue repair models. However, isolating these primary cells is complex. Each step, from bone collection and filtration to density gradient separation, can impact cell viability and purity. Key questions include: How should the appropriate density gradient medium be selected? How can stable layering be ensured? What are the critical parameters of the culture system?
In this issue of the Cell Culture Academy, we provide a comprehensive guide to isolating and culturing bone marrow monocytes, ensuring viable, stable cells for reliable downstream experiments.
Ⅰ. Basic Information
Cell Name: Mouse Bone Marrow Monocytes
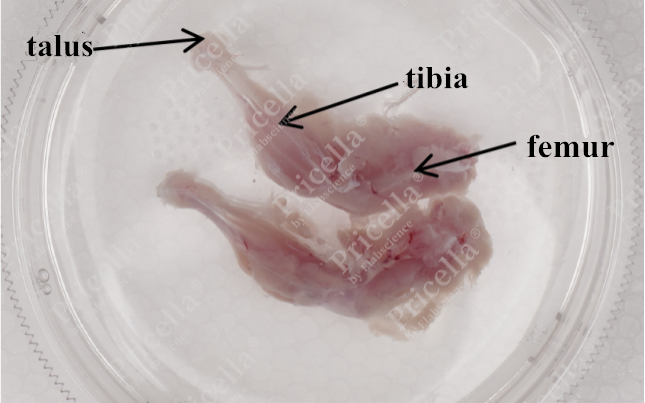
Tissue Source: Bone marrow from the femur or tibia
Cell Description: Bone marrow monocytes are myeloid precursor cells, nonproliferating cells with the potential to differentiate into macrophages, dendritic cells (DCs), or osteoclasts. They play a key role in the body's immune defense system. Despite comprising only about 0.04% of mouse bone marrow cells, they are crucial for immunoregulation. Studies show that the yield of osteoclasts differentiated from bone marrow monocytes is higher than that from peripheral blood mononuclear cells. Additionally, the osteoclast number and differentiation efficiency from whole bone marrow induction surpass those induced by splenocytes.
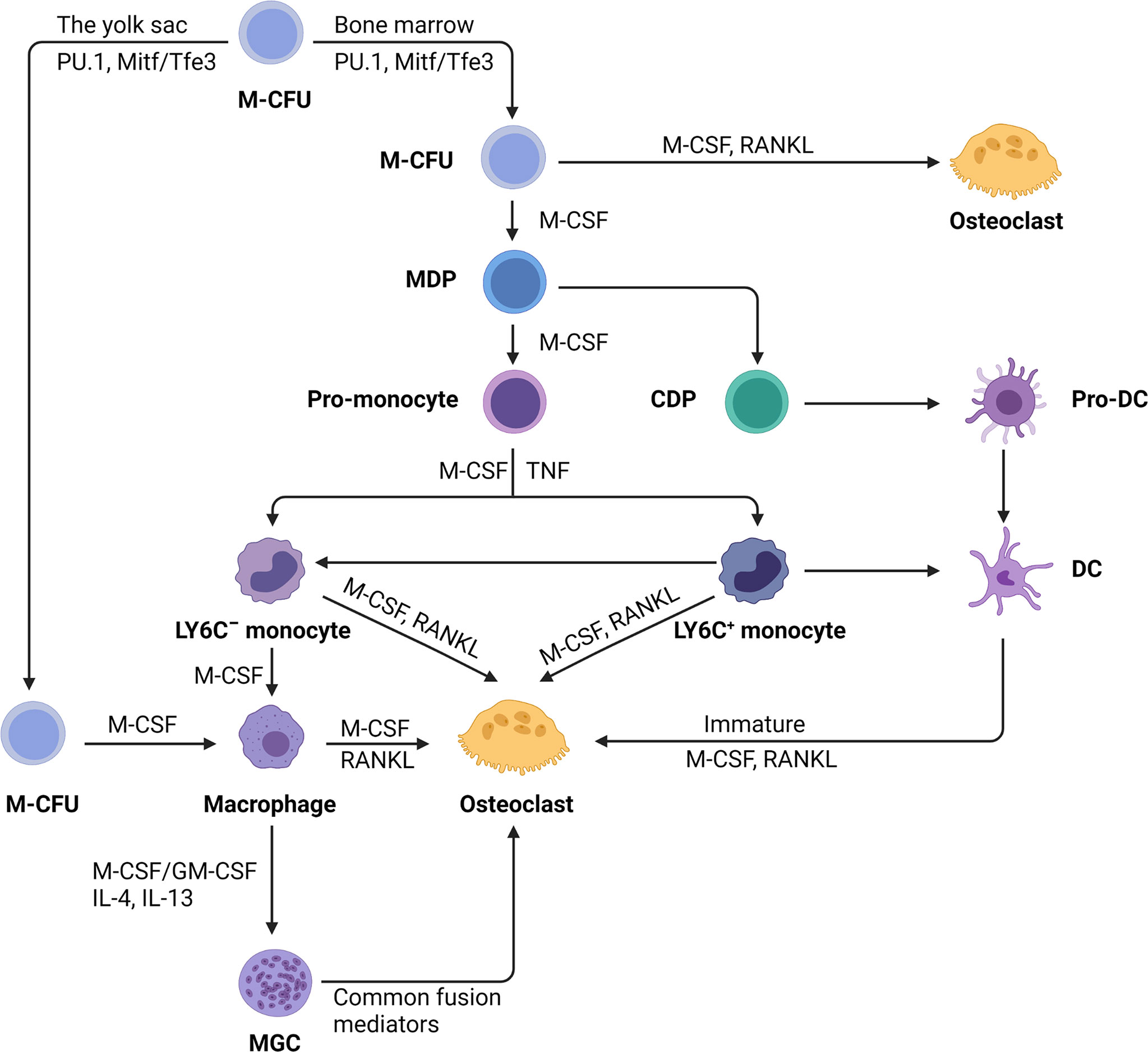
Figure 1. Differentiation of monocytes into macrophages, dendritic cells, and osteoclasts (Image from Reference 1).
Ⅱ. Operational Procedures
1.Dissection Protocol
- Euthanize 20-30-day-old C57 or Kunming mice by an overdose of pentobarbital sodium or cervical dislocation. Secure the mice on a clean bench.
- Grasp the dorsal skin, and make incisions along both sides to the abdomen, exposing the hind limbs. Retrieve the complete femur and tibia (Figure 2).
2.Tissue Processing
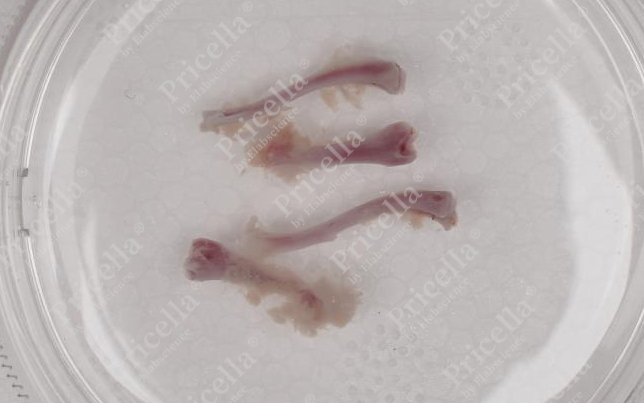
- Dislocate the joint in the opposite direction of its natural movement to separate the femur and tibia, then remove any residual muscle from the bone surface (Figure 3).
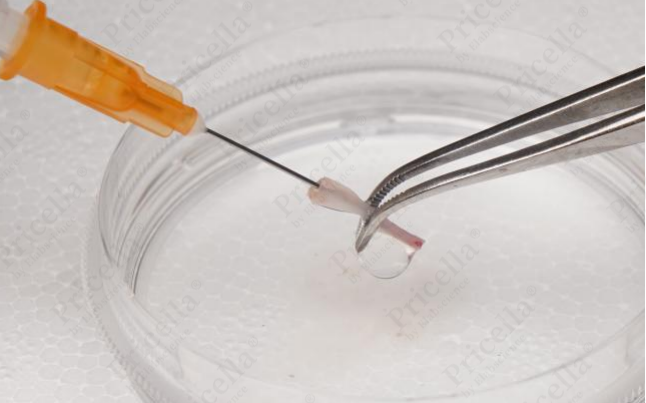
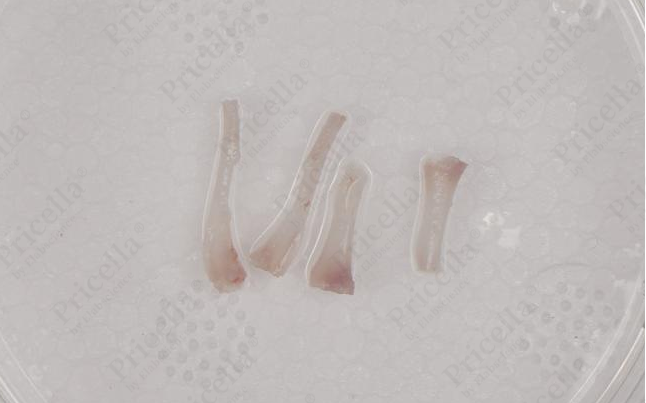
- Trim both ends of the femur and tibia. Using a syringe, insert the needle into the thick end of the bone shaft and flush repeatedly (Figure 4) until the bone becomes white and translucent (Figure 5).
- Collect the washing solution in a Petri dish and gently pipette it to obtain the bone marrow cell suspension.
3.Tissue Digestion
- Filter the bone marrow suspension through a cell filter . After centrifuging the filtrate, discard the supernatant.
- Resuspend the cells in 3 mL of PBS. In a new 15-mL centrifuge tube, add 4 mL of the special separation solution. Slowly layer the cell suspension over the surface of the separation solution along the wall of the tube to create a density gradient (Figure 6).
- Centrifuge at 1,500 ×g for 25 min with acceleration and deceleration rates set to level 1. Collect the cells at the interface between the PBS and separation solution and transfer them to a 15-mL tube. Add PBS to a final volume of 13 mL, then centrifuge at 1,800 rpm for 5 min. Discard the supernatant.
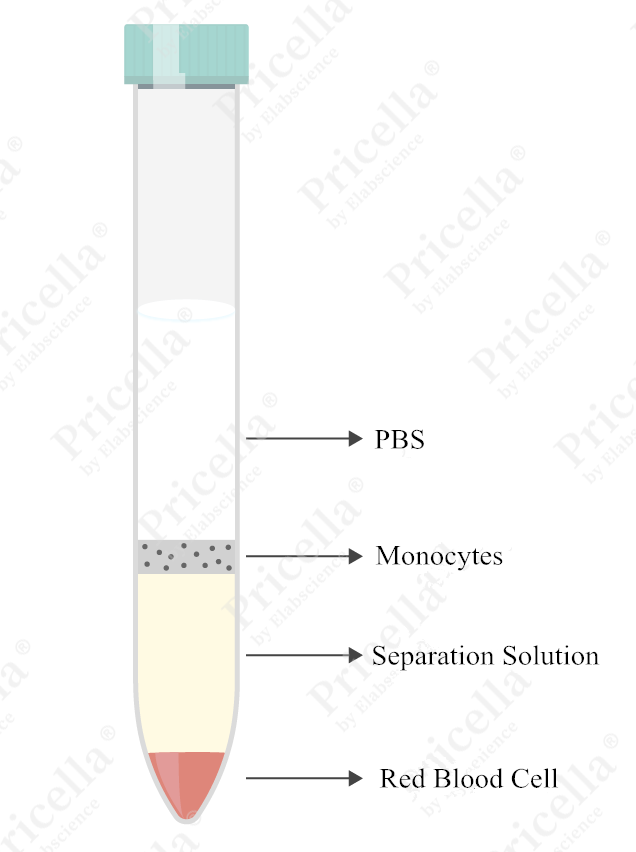
Figure 6. Reference image of cell suspension after centrifugation with Separation solution
4.Cell Culture
- Resuspend the cells in a specialized culture medium and incubate at 37°C with 5% CO2.
- Change the medium for the first time 48 h after plating. Monitor the cells under a microscope, and if confluence exceeds 80%, discard the supernatant and replace the medium.
For detailed procedures, refer to the official Mouse Bone Marrow Monocyte Isolation and Culture Kit instruction manual.
Ⅲ. Cell identification
1.Morphological Identification
The cells are either round or irregular in shape, with small prominent nuclei.
2.Marker Identification
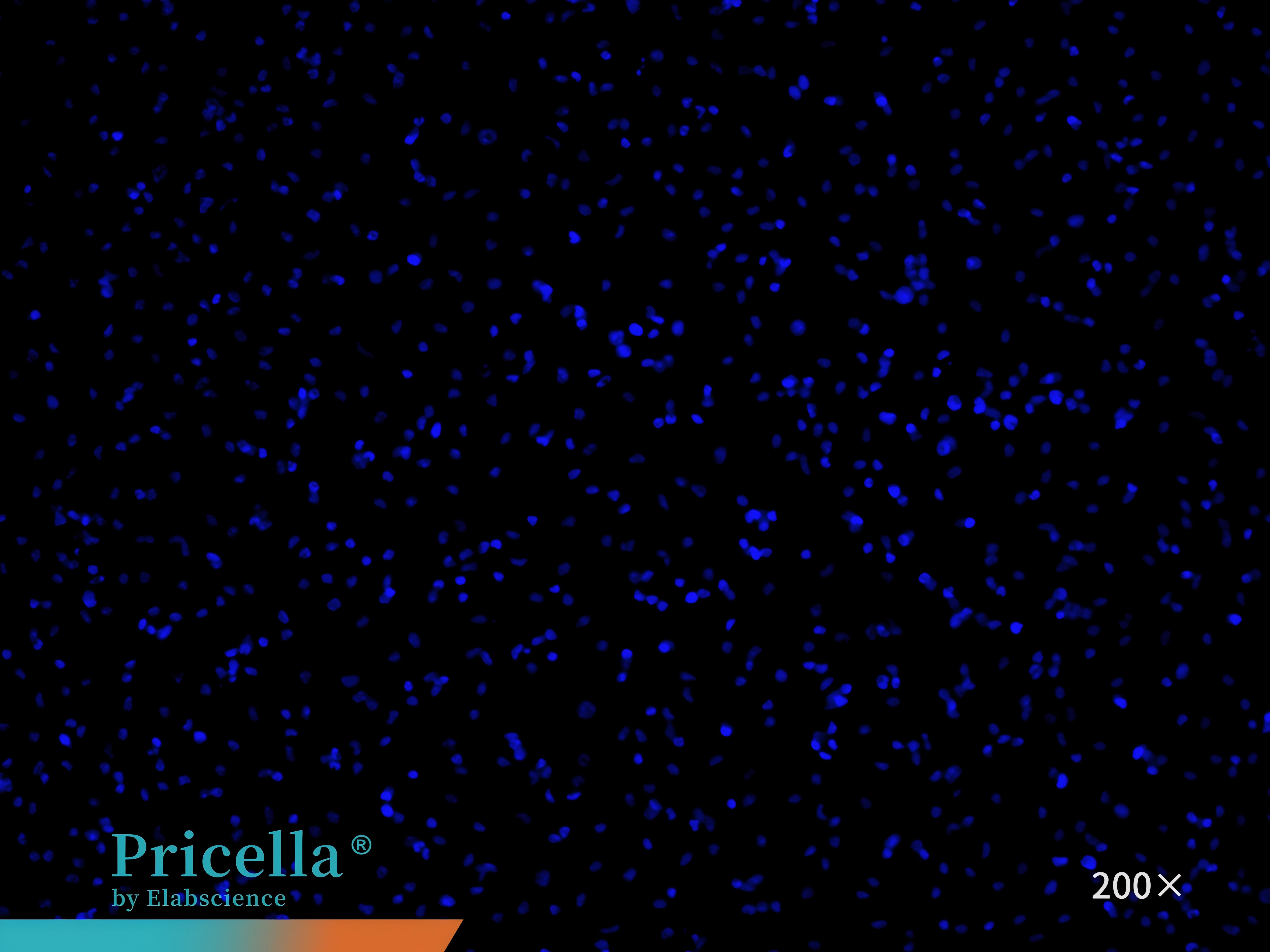
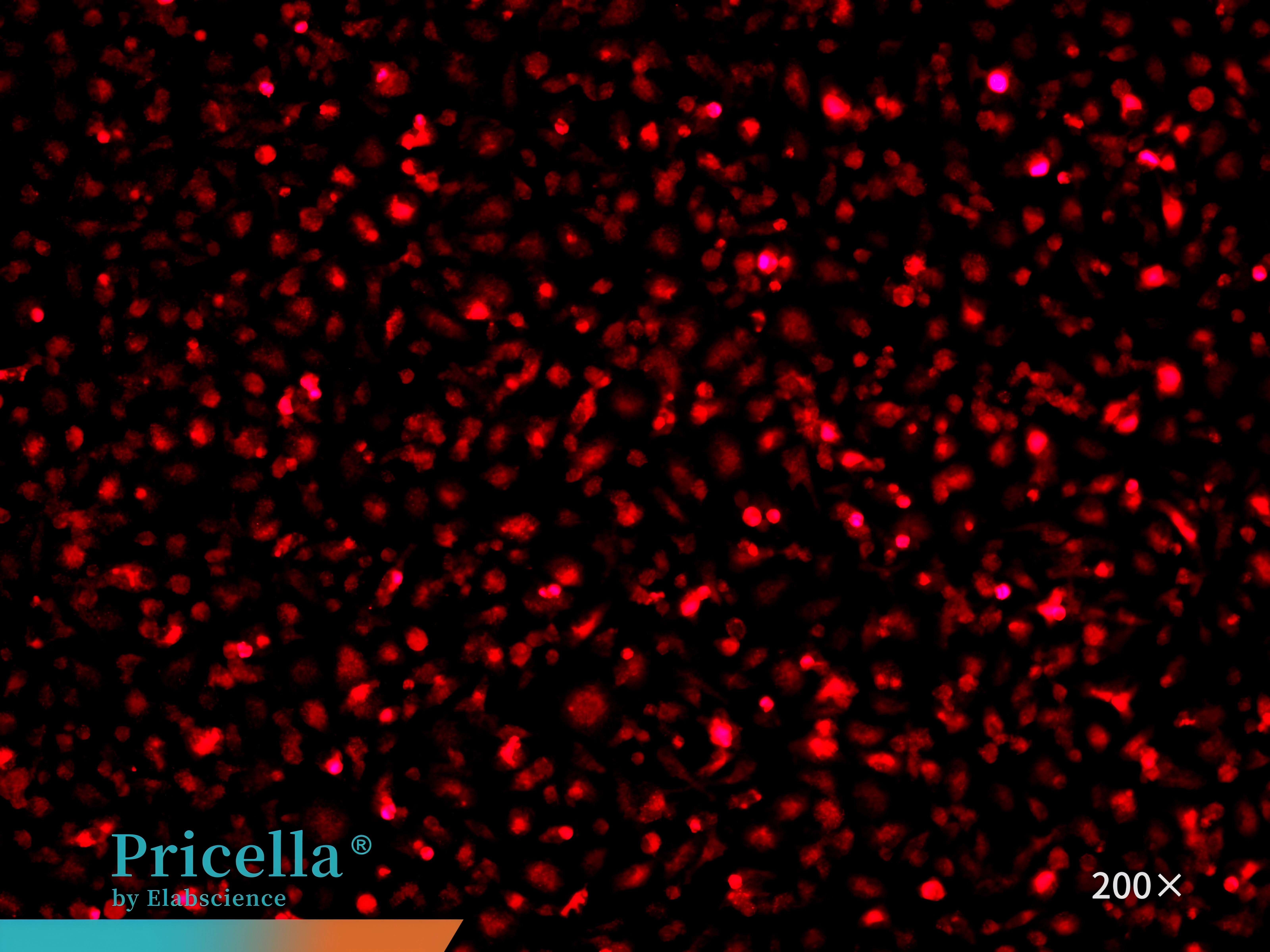
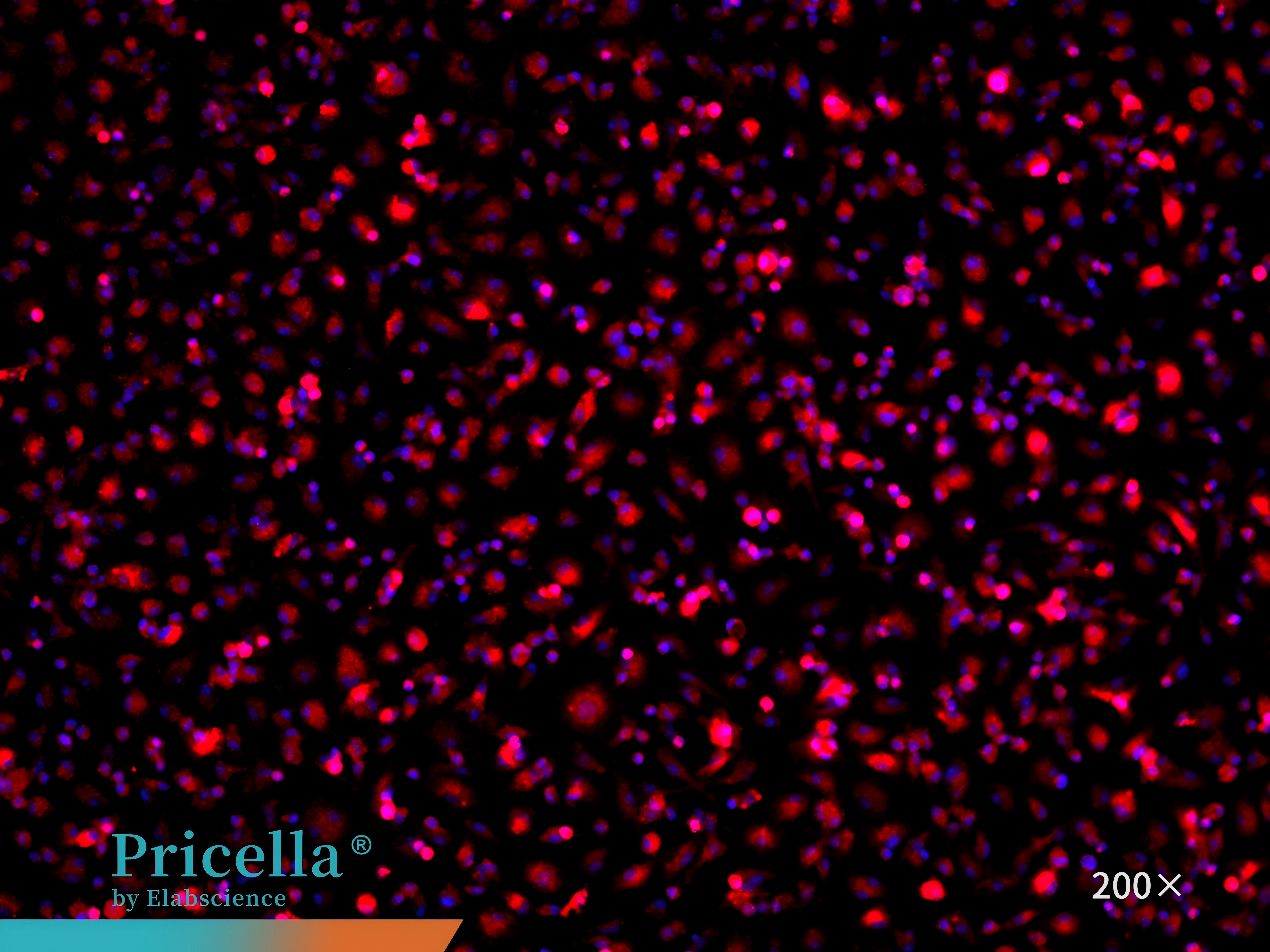
CD68 immunofluorescence staining confirms that the cell purity is greater than 90% (Figure 7).
Figure 7. Immunofluorescence identification of mouse bone marrow monocytes
IV. Culture Conditions
Culture Medium: Mouse Bone Marrow Monocyte Complete Medium
Medium Change Frequency: Every 2-3 days
Growth Characteristics: Adherent cells
Cell Morphology: Macrophage-like
Passage Characteristics: Non-proliferative; do not passage
Digestion Solution: Initially digest with lidocaine (12 mM). If cells remain undigested, switch to 0.25% Trypsin Solution . If digestion still fails, add 3 mL of complete medium to stop digestion and gently scrape the cells using a sterile cell scraper.
V. Application Directions
Bone marrow monocytes play key roles in both basic research and translational medicine.
1.Compared with peripheral blood monocytes, they are more prone to differentiate into osteoclasts and are commonly used in vitro to study bone metabolism, drug interventions, and the mechanisms underlying bone-related diseases such as osteoporosis and arthritis.
2.As myeloid precursor cells, bone marrow monocytes can migrate to tissues and differentiate into macrophages or dendritic cells, contributing to inflammation regulation, antimicrobial defense, and tissue repair. Increasing evidence shows that macrophages derived from bone marrow monocytes regulate inflammatory responses through M1/M2 polarization and are involved in the pathogenesis of diseases including nonalcoholic steatohepatitis, obesity, diabetes, and sepsis.
3. Bone marrow monocytes are also critical, dynamic regulators of the tumor microenvironment. They can be mobilized by tumors to differentiate into tumor-associated macrophages (TAMs) or myeloid-derived suppressor cells (MDSCs), thereby promoting or inhibiting tumor initiation and progression.
References:
[1] Sun Y, Li J, Xie X, Gu F, Sui Z, Zhang K, Yu T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Frontiers in Immunology. 2021 December 1; 12: 778078.
Prev: Breaking the Transfection Barrier in A549 Cells: Essential Techniques for Robust Gene Expression
Next: A No-mistake Guide to High-activity Rat Articular Chondrocyte Isolation