Targeting the Root of Cancer: A Practical Guide to Cancer Stem Cell Research
Feb 03,2026
In cancer research, a rare subpopulation of cells known as cancer stem cells (CSCs) serves as a key driver of tumor initiation, progression, metastasis, and recurrence. Although CSCs constitute only a small fraction of tumor tissues, their tumorigenic potential is significantly higher than that of bulk tumor cells. As a result, the effective identification, isolation, culture, and characterization of CSCs are critical for advancing tumor biology research and facilitating the development of novel therapeutic strategies.
In this issue of Cell Culture Academy, we systematically review the fundamental concepts of cancer stem cells, commonly used isolation approaches, tumor sphere culture and expansion strategies, and their potential applications in cutting-edge cancer research.
Ⅰ. Understanding Cancer Stem Cells
Cancer stem cells do not originate directly from the malignant transformation of normal stem cells. Instead, they constitute a rare subpopulation within tumor tissues, characterized by their capacity for self-renewal and their ability to drive tumor heterogeneity and sustain long-term growth[1]. These cells are defined by two fundamental properties.
1.Self-renewal capacity: Cancer stem cells self-renew through division, generating daughter cells that are phenotypically identical, thereby preserving the long-term stability of the tumor “seed pool”.
2. Multipotent differentiation: Cancer stem cells can give rise to multiple heterogeneous cell types within a tumor, forming its “main force”.
If a tumor is envisioned as a continuously growing “evil tree”, CSCs serve as its deeply embedded “roots”, while the bulk of differentiated tumor cells form the “branches and leaves”. Conventional radiotherapy and chemotherapy can readily remove the “branches and leaves”, shrinking the tumor but fail to eliminate the “roots”, which is a key reason many tumors develop resistance and recur.
Ⅱ. Strategies for Capturing Cancer Stem Cells
The isolation of CSCs is a prerequisite for functional and mechanistic studies. Current approaches can be broadly classified into two categories: in vitro induction and direct cell sorting.
1. In Vitro Induction
Leveraging the intrinsic plasticity of tumor cell lines, non-cancer stem cells can be induced to acquire stem-like properties under defined stress conditions. Cells obtained through induction typically require subsequent enrichment and functional validation.
A. Serum-free Culture
Serum-free media supplemented with specific growth factors, such as EGF and bFGF, are used to enrich cell populations with self-renewal capacity.
B. Hypoxic Induction
Tumor cell lines are cultured under hypoxic conditions (1-3% O2) to mimic the tumor microenvironment, resulting in the upregulation of stemness-associated genes, including OCT4 and NANOG. This approach requires precise control of oxygen levels and specialized equipment.
C. Chemotherapy Tolerance Selection
Prolonged exposure of tumor cell lines to low-dose chemotherapeutic agents, such as cisplatin or paclitaxel, selectively enriches drug-tolerant cell populations that often exhibit cancer stem cell-like features. Functional assays are required to distinguish true CSCs from nonspecific drug-resistant cells.
2. Direct Cell Sorting
Direct sorting remains the most widely used strategy for cancer stem cell enrichment. Tumor tissues are first dissociated into high-quality single-cell suspensions, followed by physical separation based on surface markers or functional properties. Among available techniques, flow cytometric sorting is considered one of the most widely used and reliable in vitro assays.
A. Surface Marker-based Sorting
Cancer stem cell markers vary across tumor types and should be selected based on published evidence[1-2]. Sorting is commonly performed using flow cytometry or magnetic bead-based methods, with efficiency largely dependent on antibody specificity.
B. Side Population Sorting
This method exploits the ability of CSCs to efflux Hoechst 33342. Although marker-independent, it requires stringent optimization of staining and detection conditions and is restricted to flow-based platforms[2].
C. Magnetic Bead Sorting
Cells are positively or negatively selected using antibody-conjugated magnetic beads. This approach is cost-effective and suitable for preliminary enrichment of large samples, although the resulting purity is generally lower than that achieved by flow cytometry.
Ⅲ. Culture and Expansion of Cancer Stem Cells
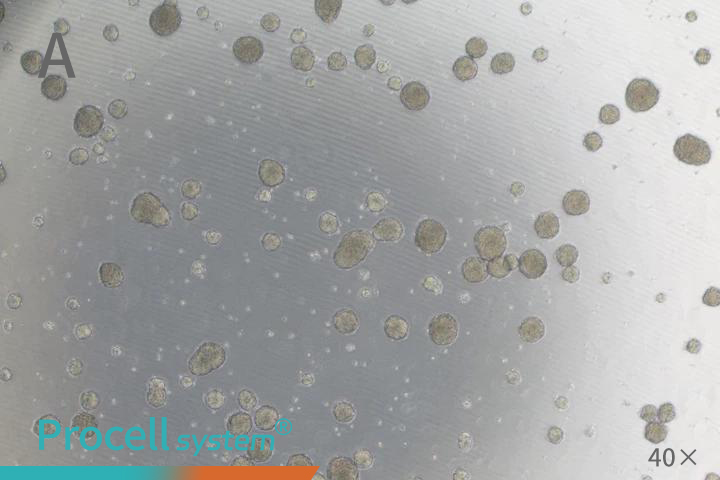
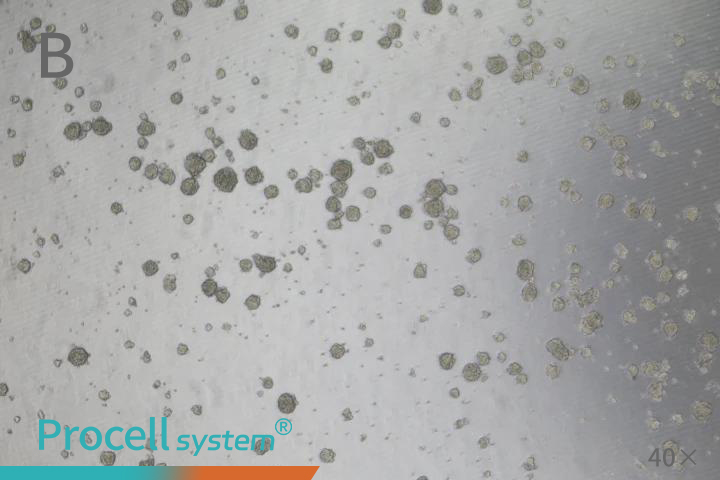
Cancer stem cells possess self-renewal capacity and can form three-dimensional spheres under serum-free, non-adherent conditions. The sphere-forming assay is currently one of the most reliable gold-standard methods for assessing stemness in vitro. Ultra-low attachment culture represents the classic approach for tumor-sphere formation.
For practical implementation, non-tissue culture-treated plastic vessels, such as ultra-low attachment plates, or vessels coated with polymers like Poly-HEMA, are commonly used. These surfaces prevent adherent growth, promote cell aggregation, and facilitate the formation of suspended spheres (Figure 1). When spheres grow too large, nutrient and oxygen limitations can cause central necrosis; therefore, subculturing is recommended once spheres are dense and uniform. During subculturing, gentle dissociation with Accutase or trypsin-EDTA yields high-quality single cells suitable for subsequent seeding.
Seeding density is critical for sphere formation. Excessive density can cause sphere fusion, whereas low density may impede sphere formation. A density-gradient assay is recommended to determine the optimal seeding concentration for each experiment.
Figure 1. A Tumor Sphere Assay for Cancer Stem Cells (A: SUM159PT; B: HCT 116)
IV. From Laboratory to Cutting-edge Applications
Stable tumor-sphere cultures of CSCs provide a versatile platform for studies ranging from basic mechanistic research to clinical translation.
1.Drug Screening and Drug-resistance Research
Conventional two-dimensional (2D) drug screens primarily target rapidly proliferating tumor cells. In contrast, three-dimensional (3D) tumor-sphere assays evaluate drug effects on CSCs, including drug penetration into the sphere core. Drug efficacy is assessed by monitoring changes in sphere size, cell viability, and stem cell marker expression.
2.Tumorigenesis and Evolution Studies
Modulating culture conditions with cytokines or small-molecule inhibitors allows dynamic tracking of CSC differentiation trajectories. Embedding tumor spheres in matrigel facilitates direct observation of microinvasion, as cells migrate into the surrounding matrix.
3. Personalized Medicine and Precision Therapy
CSCs isolated from patient tumors can be cultured into spheres for in vitro drug-sensitivity testing, enabling the identification of effective chemotherapy or targeted-drug combinations for recurrent or refractory tumors. Comparative analyses of gene and protein expression across patient-derived CSCs can reveal novel therapeutic targets and prognostic markers.
4. Tumor Microenvironment Research
Co-culture of CSCs with tumor-associated fibroblasts, immune cells, or other stromal components enables the construction of complex organoid models. These systems allow investigation of key regulatory mechanisms, including stemness maintenance and immune evasion, within the tumor microenvironment.
Prev: How Induced Differentiation Media Transforms Stem Cell Research