Analysis of Cell Culture Medium: Composition, Preparation, and Troubleshooting
Nov 27,2025
Cell culture medium serves as the essential nutrient matrix for in vitro cell cultivation, providing critical support for cell growth, proliferation, and differentiation. Composed of water, carbon sources, nitrogen sources, vitamins, minerals, and growth factors, it simulates the physiological environment of the body. This medium meets cellular metabolic demands, maintains cell viability and functional stability, and is widely used in biomedical research, drug development, vaccine production, and other fields. As an indispensable foundational material in life science experiments, it plays a vital role in scientific research.
Table of Contents
1. What are the basic building blocks of cell culture media?
2.Comparison of several common basal media
3.Factors influencing culture medium selection for different cell lines
4.How to prepare a stock solution of cell culture medium
5.Quality requirements and control standards for culture media
6.Troubleshooting common issues in cell culture media preparation
Cell culture medium is an artificially formulated nutrient matrix that sustains cell growth and reproduction. It contains essential nutrients required for cell development while providing a suitable growth environment. These synthetic media are prepared by adding specific components such as vitamins, serum proteins, carbohydrates, and cofactors to balanced saline solutions [1].
Artificially synthesized culture media are categorized into four types: serum-containing media, serum free media, chemically defined media, and protein-free media. Serum-containing media, composed of basal media supplemented with serum, is the most classical and widely used type of cell culture media. It provides essential components such as hormones, growth factors, and protease inhibitors, while neutralizing toxic substances generated during cellular metabolism. However, the use of serum presents multiple limitations, leading to the development of serum free media specifically designed for certain cell types, which employ serum substitutes or precisely defined protein components to replace serum functions. Chemically defined media and protein-free media, respectively, must meet the requirements of containing precisely defined components and being protein-free.
01 What are the basic building blocks of cell culture media?
1. Nutrient substance
● Carbohydrates: Primarily composed of glucose and galactose, they provide energy for cellular metabolism and sustain cell survival and growth. Different culture media exhibit significant variations in glucose concentration. For instance, high-glucose DMEM contains 4.5 g/L glucose, making it ideal for rapidly proliferating cells. In addition to glucose, some media incorporate sodium pyruvate as an alternative carbon source.
● Amino acids: The fundamental building blocks for cellular synthesis of proteins and nucleic acids, providing nitrogen sources and energy to sustain cell proliferation and metabolic processes. Cultures typically contain 20 amino acids, with 8 being essential amino acids that cells cannot produce through their own metabolic pathways. L-glutamine, a special amino acid, requires frequent supplementation due to its tendency to decompose in aqueous solutions [2]. Beyond supplying energy, amino acids also serve as pH buffers.
● Vitamins: Vitamins act as cofactors in cellular metabolism, providing antioxidant protection while maintaining cellular structure and function. They are essential micronutrients for cell survival, proliferation, and normal physiological activities. Common vitamins in cell culture media include B vitamins, folic acid, biotin, and vitamin C.
● Trace elements: Trace elements such as iron, zinc, copper, and selenium act as cofactors for various enzymes [3], participating in cellular processes including antioxidant defense, energy metabolism, and DNA synthesis.
2. Buffer system
● Sodium bicarbonate buffer system: The CO3/HCO3- in the culture medium and CO2 gas in the incubator form a buffer equilibrium. The equilibrium CO2 concentration required is related to the NaHCO3 concentration in the medium, typically using 5%-10% CO2 for balance [4].
● Phosphate buffer system: This system primarily functions through the HPO4²⁻/H2PO4⁻ ion exchange to form a buffer pair. While it exhibits stronger buffering capacity than sodium bicarbonate, its physiological compatibility is inferior to sodium bicarbonate. As a result, it is commonly used as a supplementary buffer in culture media. In systems without carbon dioxide, the phosphate buffer system serves as the primary buffer.
● HEPES: HEPES buffer is a synthetic amphoteric buffer, formally named 4-hydroxyethyl piperazine ethanesulfonic acid. Its molecular structure contains both proton donors (-SO3H) and proton acceptors (-N-), which maintain pH stability by neutralizing excess H+ or OH-ions in the solution [5].
● Phenol Red: Most cell culture media contain phenol red as an indicator to continuously monitor pH fluctuations. During cell growth, metabolic byproducts released by cells typically cause pH changes (usually becoming acidic), which in turn alters the medium's color. At the optimal pH range for cell culture (7.2-7.4), phenol red appears bright red. As pH decreases, the color gradually turns yellow, while an increase in pH leads to a gradual shift to purple.
● Phenol red also presents certain limitations in application: It mimics the effects of steroid hormones, particularly estrogen [6]. Therefore, phenol red-free media can be used when culturing estrogen-sensitive cells; Phenol red may interfere with flow cytometry analysis. For such research, phenol red-free media is recommended.
3. Mineral salt
Inorganic salts in culture media primarily serve to maintain osmotic balance and regulate essential ions for cellular functions. The typical salts include Na+, K+, Ca2+, and Mg2+. Na+ is the predominant ion in the extracellular fluid, directly determining cellular osmotic stability. K+ constitutes the main ion in the intracellular fluid, playing key roles in enzyme activation and pH homeostasis. Ca2+ is involved in cell adhesion, signal transduction, and muscle contraction. Mg2+ facilitates cell adhesion to the extracellular matrix and acts as a cofactor in enzymatic reactions.
4. Other additives
● Proteins and Polypeptides: Commonly added protein substances in cell culture include albumin, transferrin, fibronectin, and protease inhibitors. Albumin primarily binds and transports insoluble substances in the culture medium, while also serving as an efficient detoxifier that effectively removes toxic byproducts of cellular metabolism. Fibronectin plays a key role in cell adhesion processes. Transferrin, a transport protein, supplies iron to cell membranes. In serum-containing media, these proteins are typically added as complete serum components, whereas serum free media require concentration adjustments tailored to cellular needs.
● Lipids: As an essential component of cell membranes, lipids are naturally present in serum but require supplementation in serum free culture media.
● Growth factor: Growth factor is a kind of secreted signal molecule, which initiates intracellular signal pathway by binding to specific receptors on the cell surface. It is the key substance to maintain normal physiological state and tissue homeostasis of cells. Different types of cells need different kinds of growth factor.
Fig. 1 Cell Culture Medium Components
02 Comparison of several common basal media
MEM culture medium:
The MEM (Minimum Essential Medium) culture medium, developed by Harry Eagle in 1959 from the Eagle Basic Medium (BME), is also known as the "EMEM medium." As one of the earliest basic culture media, it contains only the essential nutrients required for cell growth, with clearly defined components and no complex natural extracts. It lacks proteins, lipids, and growth factors, requiring additional serum when used. This medium is suitable for various single-layer cultured mammalian cells, including 293, Hela, MCF-7, and L-929 cells.
Based on MEM, many kinds of culture medium such as DMEM, α-MEM, IMDM were developed (Table 1).
DMEM culture medium:
DMEM (Dulbecco's Modified Eagle Medium) is a modified version of MEM developed by Dulbecco. It contains twice the amino acid concentration and four times the vitamin concentration of MEM, with additional non-essential amino acids, trace iron ions, and sodium pyruvate. DMEM is available in two variants: high-glucose (4.5 g/L glucose) and low-glucose (1.0 g/L glucose). The high-glucose variant is ideal for rapidly proliferating and metabolically active cells such as 293T, CHO, COS-7, and RAW 264.7, while the low-glucose variant is better suited for glucose-sensitive cell lines.
IMDM culture medium:
IMDM medium (Iscove's Modified Dulbecco's Medium) was developed in 1976 by Guilber and Iscove as an enhanced DMEM medium enriched with selenium, HEPES, sodium pyruvate, and additional amino acids and vitamins, making it ideal for rapidly proliferating and high-density cultured cells. Originally designed for lymphocyte growth, studies have shown that IMDM can support mouse B lymphocytes, bone marrow hematopoietic tissues, lipopolysaccharide-stimulated B cells, T lymphocytes, hybrid cells, and other cell types [7].
Table 1. Comparison of MEM-Derived Culture Media
| Media | Key Features & Composition | Typical Applications |
| EMEM | The Standard: Based closely on the original BME formula but with higher amino acid concentrations. | Adherent Cells: Ideal for cells that form monolayers. |
| Simple: Contains only the minimum 12 essential amino acids, glutamine, and 8 vitamins. | General Maintenance: Used for a wide variety of tissue types (e.g., HeLa, fibroblasts). | |
| Buffer: Usually formulated with Earle’s salts for CO₂ incubation. | Note: Almost always requires 10% serum supplementation. | |
| DMEM | High Nutrient: Contains 4× the concentration of amino acids and vitamins found in EMEM. | Fast-Growing Cells: Supports cells with high metabolic rates. |
| Glucose: Available in Low (1 g/L) and High (4.5 g/L) glucose versions. | Broad Use: Tumor cell lines, primary fibroblasts, neurons, and hybridomas. | |
| Iron: Contains ferric nitrate (unlike IMDM). | Versatility: Good for both adherent and some suspension cultures. | |
| α-MEM | Enriched Formula: The most complex of the MEM series. | Stem Cells: The "Gold Standard" for Mesenchymal Stem Cells (MSCs) and bone marrow cells. |
| Additives: Enriched with non-essential amino acids, sodium pyruvate, lipoic acid, vitamin B12, biotin, and ascorbic acid. | Specialized Cells: Used for keratinocytes and difficult-to-culture cells. | |
| Variants: Available with or without nucleosides (ribonucleosides / deoxyribonucleosides). | Transfection: Often used during DNA transfection studies. | |
| IMDM | Super-Enriched DMEM: A highly modified version of DMEM designed for high-density culture. | Hematopoietic Cells: Specialized for Hematopoietic Stem Cells (HSCs) and macrophages. |
| Additives: Contains Selenium, HEPES buffer, sodium pyruvate, and additional amino acids/vitamins. | Immune Cells: B-lymphocytes, T-lymphocytes, and lymphoblasts. | |
| Salt Change: Uses potassium nitrate instead of ferric nitrate. | High Density: Supports rapidly proliferating high-density cell suspensions. |
M 199 medium:
Developed by Morgan in 1950, M 199 medium was the first chemically defined culture medium, originally created for nutritional studies of chicken embryo fibroblasts [8]. This medium features a unique composition including cholesterol and nucleotides such as adenine, adenosine, hypoxanthine, thymine, and various vitamins, and is widely used in primary cell culture and virus-related experiments.
Mccoy's 5A medium:
The McCoy's 5A medium, developed by Thomas McCoy and colleagues in 1959, was originally designed for long-term in vitro culture of Walker 256 cells and Novikoff hepatocellular carcinoma cells. Containing the reducing agent glutathione, bacterial peptone, and high glucose concentration, it is compatible with serum and suitable for long-term cultivation of various cell types.
RPMI 1640 medium:
RPMI 1640, a medium developed by the Roswell Park Memorial Institute (RPMI) in Buffalo, New York, is an optimized version of McCoy's 5A medium specifically designed for long-term peripheral blood lymphocyte culture. Unlike most mammalian cell culture media, its classic formulation maintains a pH of 8 (with current versions available in pH 7.2-7.4 ranges). Containing reduced glutathione and high concentrations of multiple vitamins, RPMI 1640 exhibits strong buffering capacity. When supplemented with appropriate concentrations of serum or serum substitutes, it is suitable for cultivating various cell types, including lymphocytes and hybridoma cells.
Ham's medium:
Two commonly used media are Ham's F-10 and Ham's F-12. Originally developed to support the clonal growth of CHO cells, F10 medium features a balanced nutrient profile, making it ideal for primary cells and cell lines with a steady metabolic rhythm. F12 medium, an enhanced version of F10, significantly increases the concentrations of amino acids, vitamins, and trace elements while incorporating specialized metabolites like putrescine, hypoxanthine, and thymine, effectively meeting the high-nutrient demands of challenging-to-culture cells.
DMEM F12 medium:
The DMEM F12 medium, a nutrient-rich and complex formulation, supports the growth of diverse cell types whether supplemented with serum or formulated as a serum free variant. By incorporating serum substitutes such as ethanolamine, glutathione, ascorbic acid, insulin, transferrin, serum albumin, and sodium selenite, the serum-reduced Advance DMEM F12 medium effectively reduces serum requirements during cultivation. This medium is widely used in stem cell and organoid research as a foundational culture medium.
Leibovitz's L-15 medium:
The defining feature of Leibovitz's L-15 medium is its phosphate-free buffer system, replacing the conventional sodium bicarbonate buffer. This innovation enables pH control independent of CO2 gas balance, making it ideal for short-term cell transportation, primary cell isolation, and applications where CO2 incubators are unavailable.
03 Factors influencing culture medium selection for different cell lines
1. Initial culture conditions of cells
For continuously cultured cell lines, it is advisable to maintain their original culture conditions. These conditions should be referenced from the cell manufacturer or authoritative cell banks (e.g., ATCC). Arbitrary changes to culture conditions may lead to alterations in cell phenotype. For primary isolated cells, selection should be based on their metabolic characteristics, with reference to literature for guidance.
2. Selection of cell type and source
● Species-specific requirements: Different species require distinct osmotic pressure and pH conditions for optimal cell growth. For example, insect cells thrive best at pH 6.2-6.4 and should be cultured in Grace Insect Cell Medium, while mammalian cells typically require DMEM or RPMI 1640 media.
● The metabolic characteristics of cells depend on the tissue type, such as epithelial tissue, connective tissue, nerve, hematopoietic cells, etc. Lymphoid cells (such as white blood cells) usually prefer RPMI 1640 or IMDM medium, while fibroblasts and other cells with faster metabolism should choose DMEM medium.
● Cell types: Different cell types, such as tumor cells, primary cells, and stem cells, exhibit distinct metabolic characteristics and adaptability. Tumor cells demonstrate greater vitality and metabolic activity, typically thriving in nutrient-rich media with fewer additives. Primary cells often require specialized media containing specific growth factors and hormones. Stem cell culture, however, must maintain cellular undifferentiation while ensuring normal proliferation.
3. Energy and Nutritional Needs
● Cells with high glycolytic metabolism metabolize glucose rapidly, producing substantial lactic acid. Therefore, they require a high-glucose medium with a robust buffer system, such as DMEM High Glucose Medium or IMDM Medium. Sodium pyruvate and a higher concentration of L-glutamine should also be added. Additionally, the medium must be regularly replaced to eliminate lactic acid buildup.
● Cells such as hepatocytes, adipocytes, and cardiomyocytes rely on lipid synthesis and degradation for metabolic processes. Suitable culture media include William's E medium and DMEM F12, supplemented with lipid components and antioxidants like vitamin E. Alternatively, specialized culture media can be directly used.
● Nutrition-sensitive cells, such as stem cells and primary cells, exhibit low metabolic activity but are highly sensitive to the types and concentrations of nutrients. These cells require precisely formulated growth and nutrient factors, making serum free or low-serum specialized culture media a viable option.
4. Purpose of research
● In the production of recombinant protein and vaccine, it is necessary to maximize the expression of the product, reduce the accumulation of metabolic waste, and ensure the purity and activity of the product. Generally, it is necessary to use industrial-grade special production medium, and it is necessary to optimize and adjust according to the characteristics of cell metabolism and production process, and to use serum free and chemical composition clear formula as the main.
● To induce cell differentiation, it is necessary to provide specific signal molecules, to simulate the physiological microenvironment in vivo, to select specific culture medium with clear components, to reduce the proportion of serum, or to use serum free formula directly, so as to reduce the possible inhibitory effect of serum on differentiation.
● For drug screening and drug sensitivity testing, low-serum or serum free media with clearly defined components should be prioritized. Components that may interact with drugs should be eliminated from the media. A low-glucose formulation can be used to minimize interference from metabolites, while maintaining stable pH levels in the medium.
04 How to prepare a stock solution of cell culture medium
Commercially available culture media typically come in three forms: powdered culture media, liquid culture media, and concentrated culture media. Liquid working solutions are the most convenient to use, though they have a shorter shelf life and transportation period. Concentrated culture media are used only in specific cases, such as soft agar clone cultures. Powdered culture media are the most cost-effective and easy to store, but they require filtration and sterilization during preparation.
The preparation process and precautions for powder culture medium are described below:
1. Preparation: Prepare the following equipment: beakers, magnetic stirrer, pH meter, 0.22 μm filter membrane, and reagent bottles. Add 90% volume of ultrapure water or water for injection to the beakers (e.g., 900 mL of water for preparing 1 L of culture medium). Maintain the water temperature at 20-30°C during the preparation process.
2.Weigh the powder: Based on the required volume, calculate the mass of the powder. Add the weighed powder to the beaker and stir thoroughly for at least 20 minutes until it dissolves completely.
3.pH adjustment: Add sodium bicarbonate or HEPES as specified in the manual, stir thoroughly, and adjust the pH to the target range (typically 7.2-7.4, as per the manual) using 1 M HCl or 1 M NaOH solution. After adjustment, let it stand for 5 minutes before retesting.
4.Sterilization by filtration: The medium is filtered through a 0.22 μm membrane under vacuum to remove bacteria, and the collected medium is stored in sterile vials. This step is critical for medium preparation, requiring strict aseptic conditions throughout. The filtered medium must be sealed and stored immediately.
Matters Need Attention:
● The medium should be prepared with ultrapure water or water for injection to avoid contamination by heavy metals and endotoxins.
● Add water before adding the powder to prevent clumping and ensure proper dissolution.
● Avoid high temperatures (above 40℃) that may destroy the nutrients in the culture medium.
● The medium should be filtered and sterilized as soon as possible after dissolution to avoid microbial proliferation and endotoxin elevation under non-sterile conditions.
05 Quality requirements and control standards for culture media
1. Contamination-free
The absence of contamination in culture media constitutes the fundamental requirement. Standard testing typically includes bacterial and fungal contamination, while for media derived from natural sources such as serum, additional detection of viruses and mycoplasma is necessary. Endotoxins, as residual metabolic byproducts of bacterial contamination, determine the endotoxin levels in final products based on contamination levels in raw materials, water, and the production process (prior to filtration and sterilization). These endotoxins cannot be eliminated through filtration alone but must be minimized through process control. The endotoxin level in cell culture media should be maintained below 3 EU/mL.
2.The physical and chemical properties are stable
● Stable pH: The pH level should remain stable (typically 7.2-7.4, as specified in the medium manual) when stored at room temperature or 4°C. Note that after opening, the medium's pH may shift due to low CO2 concentration in the air (often turning purple). Therefore, opened media should be used promptly or aliquoted to minimize opening frequency.
● Optimal osmotic pressure: Animal cell culture media typically maintain an osmotic pressure of 280-320 mOsm/kg, matching intracellular levels to prevent cell shrinkage or swelling caused by osmotic imbalance. Note that adding high-concentration substances or excessive evaporation may elevate osmotic pressure. Therefore, it's crucial to control additive concentrations, maintain saturated humidity in incubators during cultivation, minimize medium evaporation, and regularly replace the medium with fresh stock.
● Storage stability: During storage, the nutritional components remain intact with no significant pH changes, and the performance remains consistent throughout the shelf life. The most susceptible component in culture media is L-glutamine, which degrades gradually over time. For long-term storage, L-alanyl-L-glutamine can be used as an alternative to enhance the stability of the culture medium.
3.Cell growth effect
The assessment of cell growth in culture media is conducted by evaluating the cultivation performance of designated cell lines. Vero cells are commonly used for testing. Without serum supplementation, the cells maintain stable morphology without floating or death. When serum is added, it stimulates cell proliferation, enabling the cell population to reach the specified doubling time.
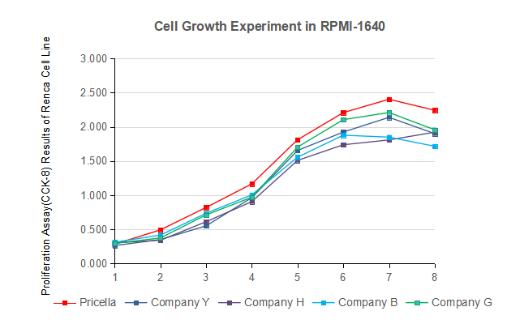
Fig. 2 Test of growth effect of RPMI-1640 cell culture medium
06 Troubleshooting common issues in cell culture media preparation
1. Powder culture medium is difficult to dissolve.
Possible reasons: The powder was poured in one go, resulting in clumping, and the mixing was not sufficient.
Solution: First, add 90% of the water volume to the container, then slowly add the culture medium powder while stirring. Adjust the stirrer speed to ensure the agitator is in the correct position.
2. The color of the medium was abnormal after preparation.
Possible causes: A yellowish color may indicate low pH or bacterial contamination, while a purplish tint typically results from elevated pH levels. This is often due to the use of vacuum filtration devices during sterilization, which reduces the concentration of HCO3- ions in the culture medium and consequently raises the pH value.
Solution: Check the medium for contamination first. If contaminated, discard and reconstitute. If not contaminated, adjust the pH to normal color with sterile 1 M NaOH if low, or with 1 M HCl if high.
3. The cells were lysed and died directly due to abnormal osmotic pressure.
The powder quantity may be inaccurate, or the mixture was not diluted to the required volume. Re-dilute and weigh accurately, then dilute as required.
References:
[1] Morgan J, Morton H, Parker R. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950, 73:1-8
https://pubmed.ncbi.nlm.nih.gov/15402504/
[2] The concentrations of glutamine and ammonia in commercially available cell culture media, S. Heeneman; N. E. Deutz; W. A. Buurman, 1993, Journal of Immunological Methods, 166(1):85–91,doi:10.1016/0022-1759(93)90331-Z
https://pubmed.ncbi.nlm.nih.gov/8228290/
[3] Sternberg J, Benoit J, Mercier A, PAQUETTE J. ROLE OF SOME TRACE ELEMENTS (ZINC AND COBALT) IN THE GROWTH OF B.C.G. Rev Can Biol. 1964;23:353-65 https://pubmed.ncbi.nlm.nih.gov/14237169/
[4] Howorth P.[4] Howorth P. The physiological assessment of acid-base balance. Br J Dis Chest. 1975, 69:75-102 https://pubmed.ncbi.nlm.nih.gov/237527/
[5] Shipman C. Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proc Soc Exp Biol Med. 1969, 130:305-10 https://pubmed.ncbi.nlm.nih.gov/5762514/
[6] Berthois Y, Katzenellenbogen J, Katzenellenbogen B. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986, 83:2496-500
https://pubmed.ncbi.nlm.nih.gov/3458212/
[7] Gupta A, Singh J, García Valverde A, Serrano C, Flynn D, Smith B. Ripretinib and MEK Inhibitors Synergize to Induce Apoptosis in Preclinical Models of GIST and Systemic Mastocytosis. Mol Cancer Ther. 2021.
https://pubmed.ncbi.nlm.nih.gov/33947686/
[8] Morgan, J. F.,Morton, H. J., & Parker, R. C. (1950). Nutrition of Animal Cells inTissue Culture. I. Initial Studies on a Synthetic Medium.,. Proceedings of the Society for Experimental Biology and Medicine, 73(1), 1-8.
https://pubmed.ncbi.nlm.nih.gov/15402504/


