Cardiac Endothelial Cell Isolation Keeps Failing? Copy This High-purity Playbook
Jan 28,2026
Cardiac microvascular endothelial cells (CMECs) are central to cardiovascular research. As key regulators of microcirculatory homeostasis, functional and pathological alterations in CMECs directly influence cardiomyocyte behavior. In addition, CMECs are primary targets of toxins, inflammatory mediators, and viral infections, making them essential for in vitro models of myocardial ischemia-reperfusion injury and endothelial-cardiomyocyte paracrine signaling.
Despite their importance, the isolation and culture of CMECs remain technically challenging. The dense, extracellular matrix-rich, and highly heterogeneous nature of cardiac tissue often results in either markedly reduced cell viability or fibroblast overgrowth when conventional digestion and isolation methods are applied, limiting the acquisition of high-purity endothelial populations.
In this issue of Cell Culture Academy, we present a reproducible, high-yield workflow for the isolation and culture of mouse CMECs, providing a reliable and standardized cell source for cardiovascular research.
I. Basic Information
Cell name: Mouse cardiac microvascular endothelial cells
Tissue source: Cardiac tissue
Cell description:
Cardiac microvascular endothelial cells form a monolayer of flattened, epithelial-like cells lining the lumen of cardiac microvessels and constitute the vascular inner wall. Through the production and secretion of bioactive factors, they play essential roles in maintaining vascular tone, regulating blood pressure, and preventing thrombosis, and are therefore of significant pathophysiological relevance in cardiovascular diseases. In addition, these cells exert both physiological and pathological effects on cardiomyocyte function, primarily by releasing nitric oxide (NO), endothelin-1, prostaglandins, purines, natriuretic peptides, and other mediators that modulate cardiomyocyte activity.
Ⅱ. Operational Procedures
Cardiac tissue is subjected to a two-step enzymatic digestion, followed by density gradient centrifugation for cell enrichment and selective expansion in an endothelial cell-specific culture medium.
1.Dissection Protocol
Mice aged 15-20 days are euthanized in accordance with approved protocols, disinfected, and placed supine on a dissection board.
The thoracic skin and underlying connective tissue are incised, the diaphragm is transected, and the manubrium is cut to open the thoracic cavity and expose the heart.
Cut open the blood vessels connected to the heart, and gently excise the heart with curved forceps and immediately transferred to an ice-cold washing solution.
2.Tissue Processing
Surface blood and connective tissue are removed from the heart, followed by thorough washing.
Transfer the tissue to a 1.5 mL EP tube containing Mouse Cardiac Microvascular Endothelial Cell Complete Medium and minced into 1 mm³ pieces.
The pieces are transferred to a centrifuge tube, washed with Specialized Washing Solution 5 times, centrifuged at 300 g for 1 min, the supernatant discarded, and the tissue precipitate retained.
3.Tissue Digestion
Digestive Solution A is added to a centrifuge tube, and the mixture is incubated at 37°C in a shaking water bath (150 rpm) for 1 h.
Digestive Solution B is then added, and incubation continues under the same conditions for 30 min.
After digestion, the tissue is thoroughly mixed with a dedicated Washing Solution, filtered through a cell strainer, and the filtrate is collected.
4.Cell Isolation
Initial centrifugation: The filtrate is centrifuged at 300 g for 5 min. Discard thesupernatant and retain the precipitate
Washing and resuspension: The pellet is resuspended in 5 mL Washing Solution and centrifuged again at 300 g for 5 min. The supernatant is discarded, and the pellet is resuspended in 3 mL Specialized Isolation A to generate a cell suspension.
Gradient preparation: In a new 15 mL tube, 5 mL of Specialized Isolation B is added. The cell suspension is gently layered on top to form a distinct interface.
Density gradient centrifugation: Centrifuge at 900 g for 15 min (set the acceleration to 1 and the decelerate to 0)
Collection of the white membrane layer: After centrifugation, a visible layer of microvascular segments forms at the interface between Specialized Isolation Solution A and B (Figure 1,). This layer is carefully collected and transferred to a new tube.
Cell washing: Add Washing Solution resuspend white membrane cells, centrifuged at 300 g for 5 min. Discard the supernatant and retain the precipitate.
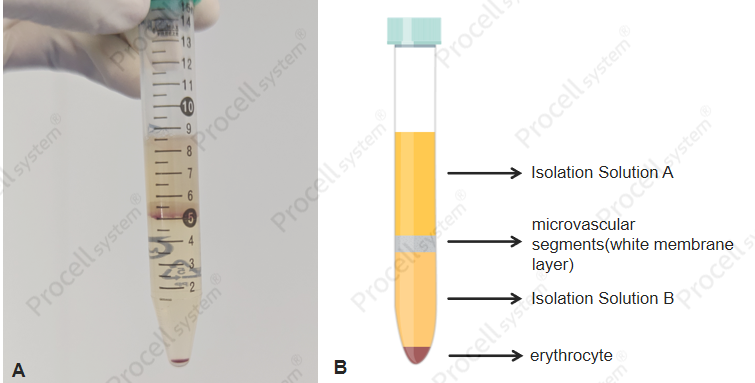
Figure 1. Purification of cells by separation solution (A: Real image; B: Schematic diagram)
5.Cell Culture
Coating: Add the Planting Solution to a T25 culture flask and incubate at 37℃ for 0.5-2 h.
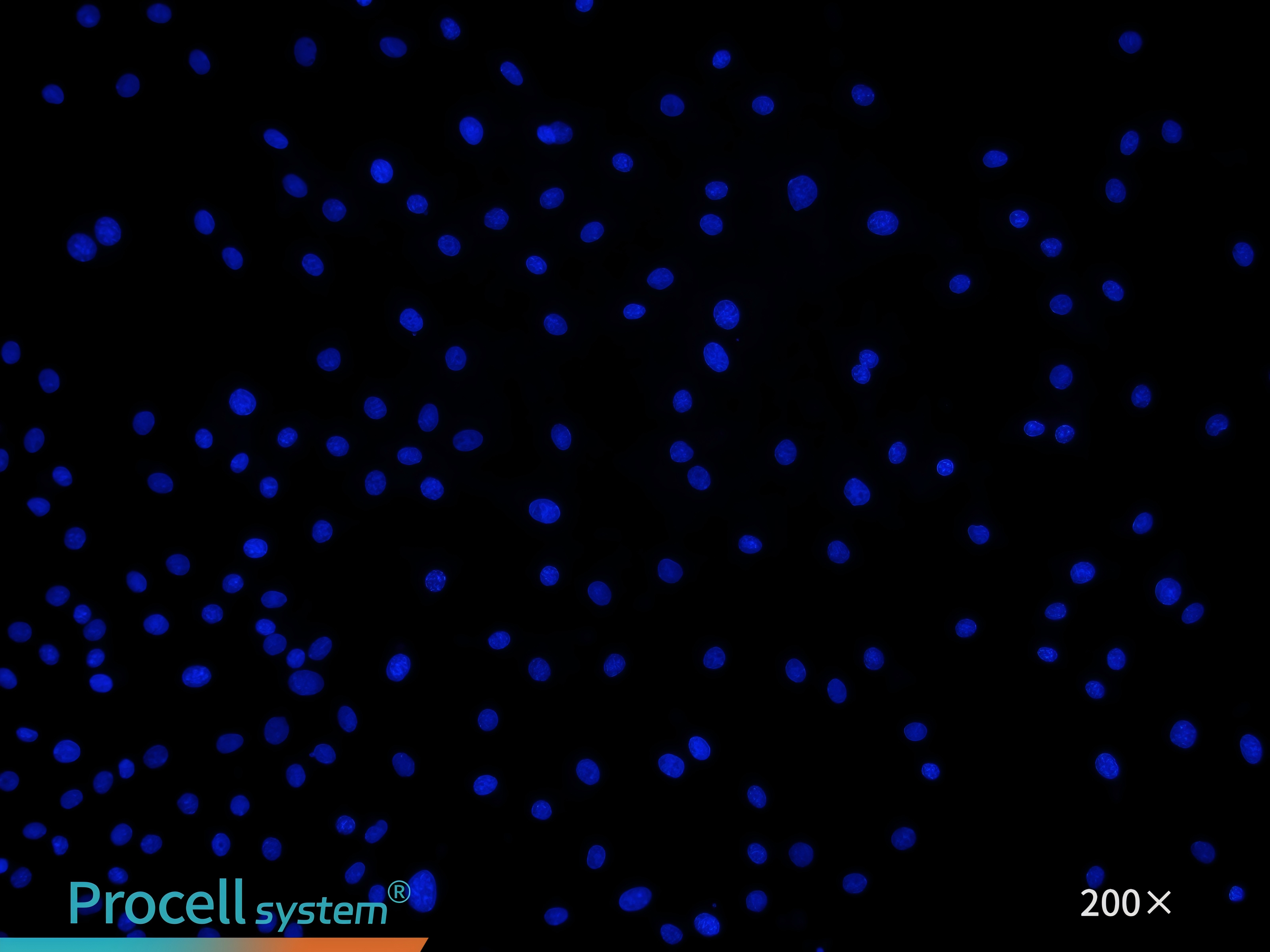
Cell seeding: Seed mouse cardiac microvascular endothelial cells into the coated flask. After seeding, microvascular fragments are visible floating in the medium (Figure 2).
Medium replacement: After 48 h of culture at 37°C with 5% CO2, the medium above adherent cells contains numerous dead cells, debris, and erythrocytes. Carefully aspirate the supernatant, gently rinse the flask with PBS, and tap the bottom to dislodge residual debris. Discard the PBS and add fresh complete medium. Subsequently, replace the medium every 2-3 days.
Cell passaging: When cultures reach 80-90% confluence, initiate passaging. Add 1 mL Accutase Cell Detachment Solution and incubate for 5 min. Collect the cell suspension and centrifuge at 300 g for 5 min. Retain the pellet and resuspend in complete medium.
For detailed stepwise instructions, refer to the Mouse Cardiac Microvascular Endothelial Cell Isolation and Culture Kit manual.
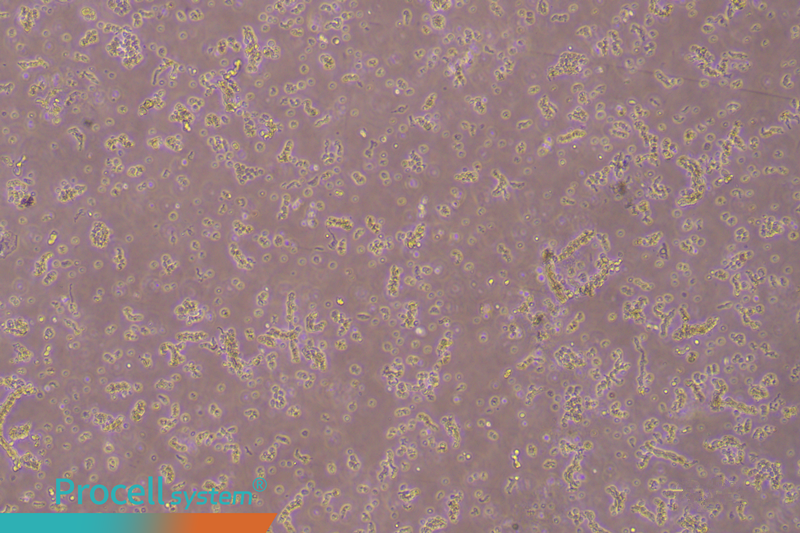
Figure 2. The cell suspension immediately after inoculation shows distinct microvascular tissue
Ⅲ. Cell Characterization
1. Morphology
Cells exhibit a typical “cobblestone” morphology, displaying polygonal and spindle-shaped forms, with large, clearly defined nuclei.
2. Marker Analysis
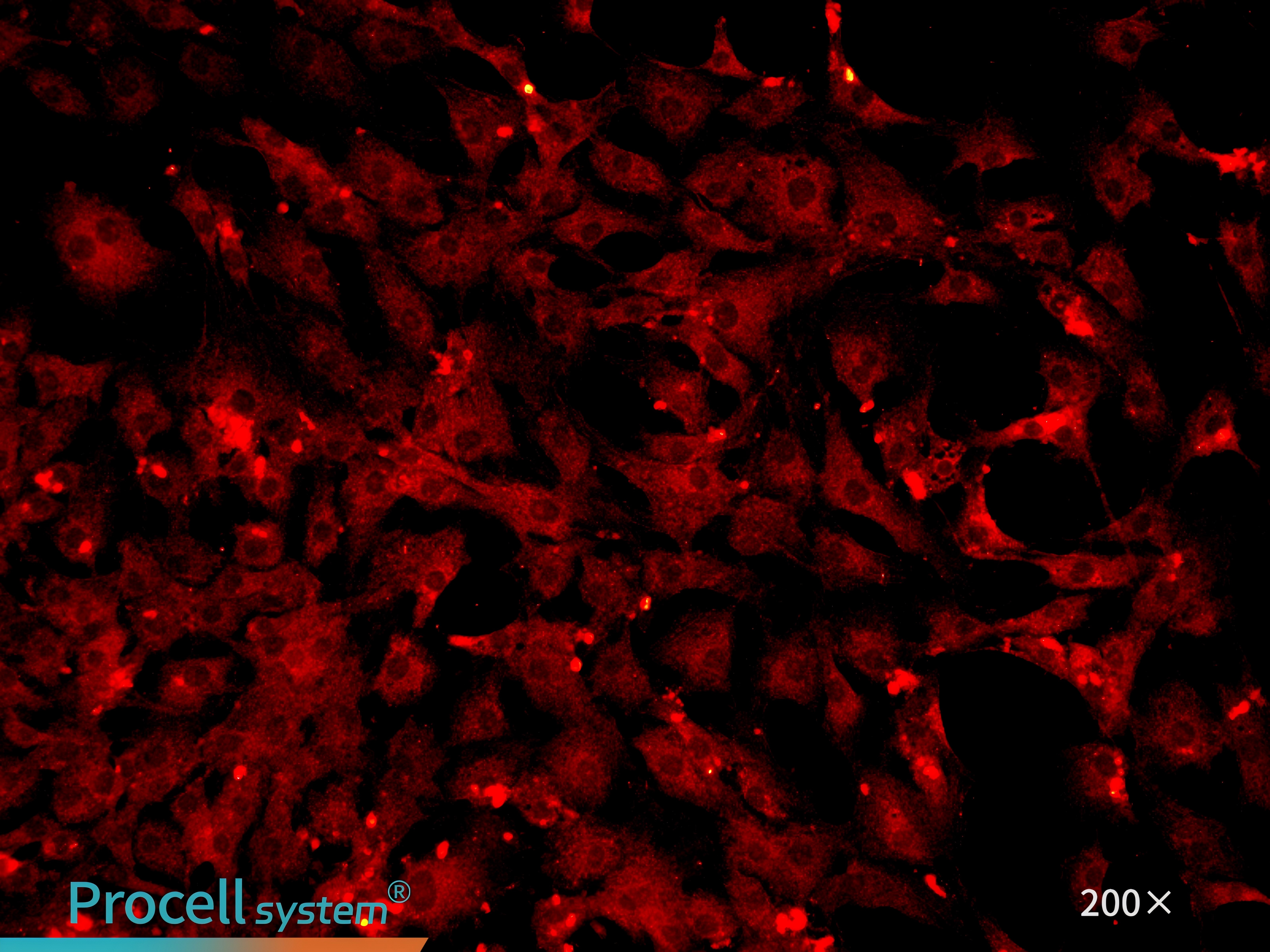
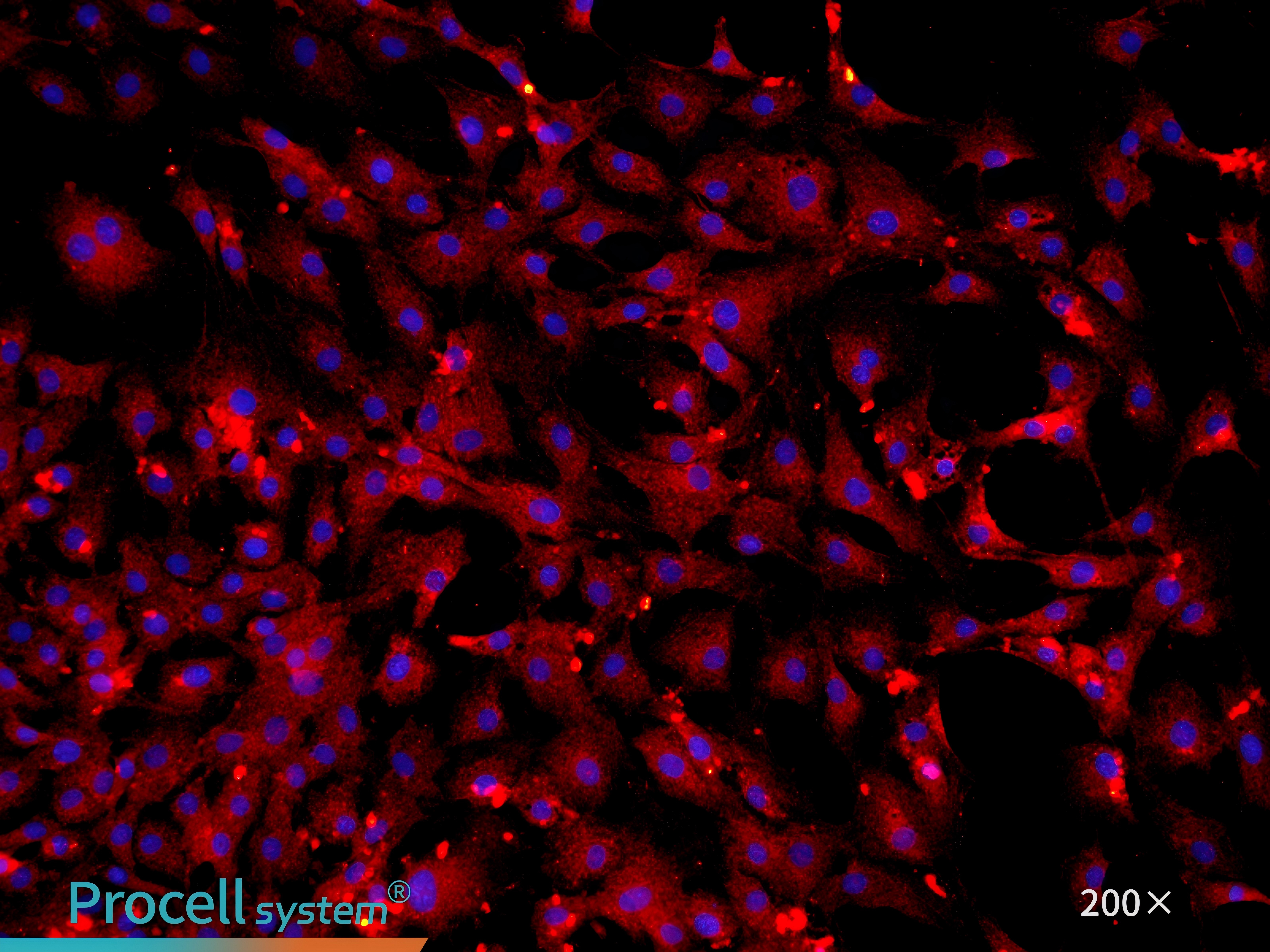
Immunofluorescence staining for the endothelial celll-specific marker CD31 confirms cell purity of 90% (Figure 3).
Figure 3. Immunofluorescence identification of mouse cardiac microvascular endothelial cells
IV. Culture Conditions
Coating: Poly-L-Lysine Solution (0.1 mg/mL) or Gelatin Coating Solution (0.1%)
Culture medium: Mouse Cardiac Microvascular Endothelial Cell Complete Medium
Medium change Frequency: Every 2-3 days
Morphology: Adherent, epithelial-like cells
Passage ratio:1:2
Passage characteristics: Can be passaged 2-3 times
Digestion: Accutase Cell Detachment Solution
V. Applications
1. Cardiac Ischemia-Reperfusion Injury
CMECs is a key contributor to acute cardiomyocyte death and a hallmark of ischemia-reperfusion injury (IRI). Myocardial ischemia disrupts endothelial barrier integrity, increases vascular permeability, and triggers inflammatory mediator release, while reperfusion amplifies oxidative stress and inflammation. Hypoxia-reoxygenation treatment of CMECs provides an in vitro model to study endothelial dysfunction induced by restored blood flow, enabling investigation of mechanisms such as inflammation, oxidative stress, and signaling pathway dysregulation[1].
2. Diabetic Cardiomyopathy
Microvascular injury is an early and critical event in diabetic cardiomyopathy (DCM). High-glucose and high-lipid exposure of CMECs can model diabetes-associated cardiac alterations in vitro. MICU1 deficiency has been shown to cause excessive mitochondrial calcium uptake and homeostatic imbalance, leading to nitrosative stress, endothelial injury, and inflammation, which impair CMEC barrier function and drive DCM progression[2].
3. Angiogenesis and Vascular Repair
CMECs are widely used to study myocardial angiogenesis and vascular repair. Co-culture with cardiac telocytes (CTs) demonstrates that miRNA-21-5p in CT-derived extracellular vesicles targets the p53 downstream gene Cdip1, reducing activated caspase-3 levels. This inhibits CMEC apoptosis and promotes angiogenesis and tissue regeneration following myocardial infarction[3].
References:
1.Ma L, Zou R, Shi W, et al. SGLT2 inhibitor dapagliflozin reduces endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury through normalizing the XO-SERCA2-CaMKII-coffilin pathways. Theranostics. 2022;12(11):5034-5050.
2.Shi X, Liu C, Chen J, et al. Endothelial MICU1 alleviates diabetic cardiomyopathy by attenuating nitrative stress-mediated cardiac microvascular injury. Cardiovasc Diabetol. 2023;22(1):216.
3.Liao Z, Chen Y, Duan C, et al. Cardiac telocytes inhibit cardiac microvascular endothelial cell apoptosis through exosomal miRNA-21-5p-targeted cdip1 silencing to improve angiogenesis following myocardial infarction. Theranostics. 2021;11(1):268-291.
Prev: Overcoming Low Transfection Efficiency in THP-1 Cells Through mRNA Delivery
Next: How Induced Differentiation Media Transforms Stem Cell Research